Abstract
For many autoimmune diseases, the underlying mechanism is still unknown. In order to get more insight into the etiology of autoimmune diseases, we recently published a study were we performed epigenetic profiling and RNA sequencing on CD4+CD45RO+ T cells derived from the site of inflammation of Juvenile Idiopathic Arthritis (JIA) patients and compared this with healthy controls [1]. In this “Data in Brief”, we focus on the analysis of our RNA sequencing data reported in this study, of which the raw and processed files can be found in GEO under GSE71595. We provide a detailed description of the downstream analysis, quality controls, and different analysis methods or techniques that validate the results obtained with RNA-sequencing.
Keywords: Autoimmune disease, RNA-sequencing, Juvenile Idiopathic Arthritis, BET-inhibition
| Specifications | |
|---|---|
| Organism/cell line/tissue | Homo sapiens/CD4+CD45RO+ T cells/synovial fluid (SF) and peripheral blood (PB) |
| Sequencer or array type | Illumina NextSeq500 sequencer |
| Data format | Raw and analyzed |
| Experimental factors | Healthy control (HC)-derived cells obtained from PB vs. Juvenile Idiopathic Arthritis (JIA) patient-derived cells obtained from SF. |
| Experimental features | CD4+ CD45RO+ cells were sorted from HC peripheral blood mononuclear cells (PBMC) and JIA synovial fluid mononuclear cells (SFMC), and cultured for 16 h in the presence of CD3/CD28 dynabeads and 300 nM JQ1(+) or JQ1(−). Total RNA was extracted, followed by next-generation sequencing to study global transcriptome differences between HC and JIA patients and to assess the effect of JQ1(+) on gene expression in JIA patient-derived T cells. |
| Consent | Informed consent was obtained from all patients either directly or from parents/guardians when the patients were younger than age 12 years. |
| Sample source location | University Medical Center Utrecht, Utrecht, The Netherlands. |
1. Direct link to deposited data
The deposited data can be found at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE71595.
2. Experimental design, materials and methods
2.1. Study population and sample collection
Four HC and three oligoarticular JIA patients were included who all had active disease at the time of sampling. SF was collected upon therapeutic joint aspiration and PB was drawn at the same moment. SFMCs and PBMCs were isolated using Ficoll Isopaque density gradient centrifugation and were frozen down in FCS containing 10% DMSO.
2.2. RNA sample preparation and sequencing
CD4+CD45RO+ cells (ranging between 600.000–1.200.000 cells per sample) were isolated from frozen HC PBMCs and JIA SFMCs using flow cytometry, activated with human T-activator CD3/CD28 dynabeads (1 cell: 3 beads) and cultured for 16 h in the presence of 300 nM JQ1(+) or JQ1(−). Subsequently, cells were treated for 4 h with phorbol 12-myristate 13-acetate (100 ng/ml) and ionomycin (1 μg/mL). Total RNA was extracted using the RNAeasy kit (Qiagen), according to the manufacturer's instructions. The quality of the RNA was assessed based on the RNA profile generated by the bioanalyzer using the picochip. Removal of rRNA and globin-encoding mRNA, RNA fragmentation, cDNA generation, adapter ligation and PCR amplification was done using the TruSeq stranded total RNA with ribo-zero globin sample preparation kit (Illumina) and 75 bp single-end sequencing was performed using an Illumina NextSeq 500 platform through the Utrecht DNA sequencing facility.
2.3. Quality control
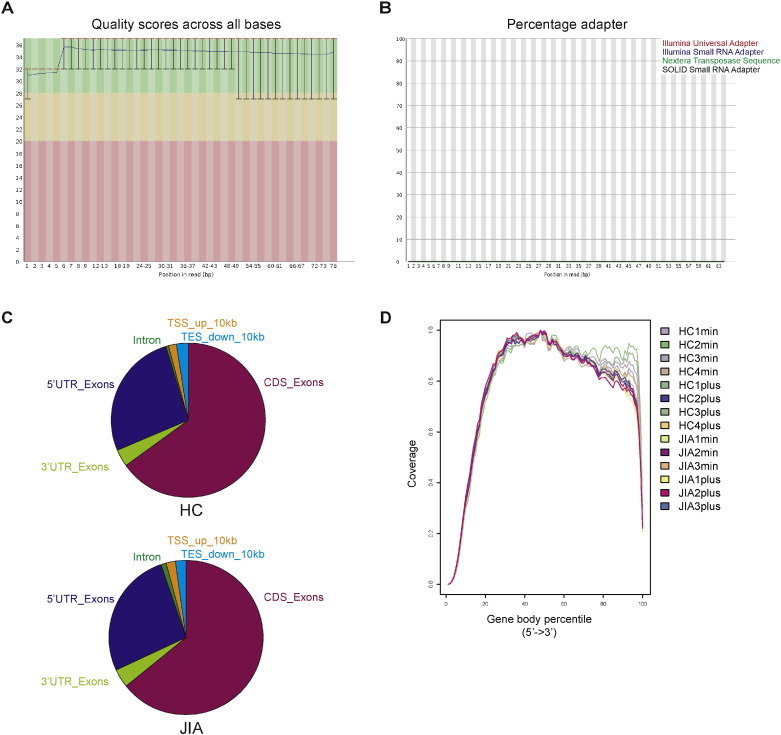
Read quality assessment, sample demultiplexing, and adapter trimming was performed by Utrecht DNA sequencing facility using BaseSpace (Illumina) software. Reads with quality score of Q > 30 were selected for downstream analysis. Before starting downstream analysis, FastQC (Babraham Bioinformatics) was used to confirm previous steps (Fig. 1A and B). As a human reference genome, hg19 was used, and reads were mapped to this reference sequence using Tophat v.2.0.9 [2] with Bowtie 2.1.0 [3] as index (tophat2 -p 8 –library-type fr-firststrand –g hg19.gtf). For each sample ± 91% of the reads were successfully mapped to the genome, indicating sufficient coverage. RSeQC was used to calculate how mapped reads were distributed over DNA regions, such as coding sequences and UTRs, and to assess uniform read coverage of genes [4] (Fig. 1C and D).
Fig. 1.
Quality control steps in RNA-sequencing data analysis. (A) Read quality score across all bases, representative image of one sample is shown. (B) Percentage adapters in reads, representative image of one sample is shown. (C) Average percentage of read distribution over DNA regions from untreated 4 HC and 3 JIA samples (read distribution is also representative for JQ1(+) treated samples). (D) Read coverage of housekeeping genes for all samples.
2.4. Gene expression analysis and validation
Transcriptomes of each individual sample were generated using CuffLinks v2.2.1 with hg19 gene annotation as a guide [5], [6]. Individual transcripts belonging to the HC or JIA patient group were merged using Cuffmerge. The resulting transcriptome was used for differential gene expression analysis using Cuffdiff. Quartile normalization was performed (–library-norm quartile) to improve the detection of differential expression of less abundant transcripts. Reads mapping to rRNA and tRNA were masked (-M option) from the quantification. Cuffnorm was used to generate quartile normalized count and FPKM tables. Using an FDR ≤ 0.05, 914 transcripts were identified that are significantly differentially expressed between HC and JIA patients. Among these transcripts, 637 were upregulated in JIA patients compared to HC, while 237 were downregulated in JIA patients. JQ1(+) and JQ1(−) treatments of JIA patient-derived memory/effector T (Tmem/eff) cells resulted in 633 differential transcripts, based on FDR ≤ 0.05, of which 338 were downregulated upon JQ1(+) treatment and 295 upregulated.
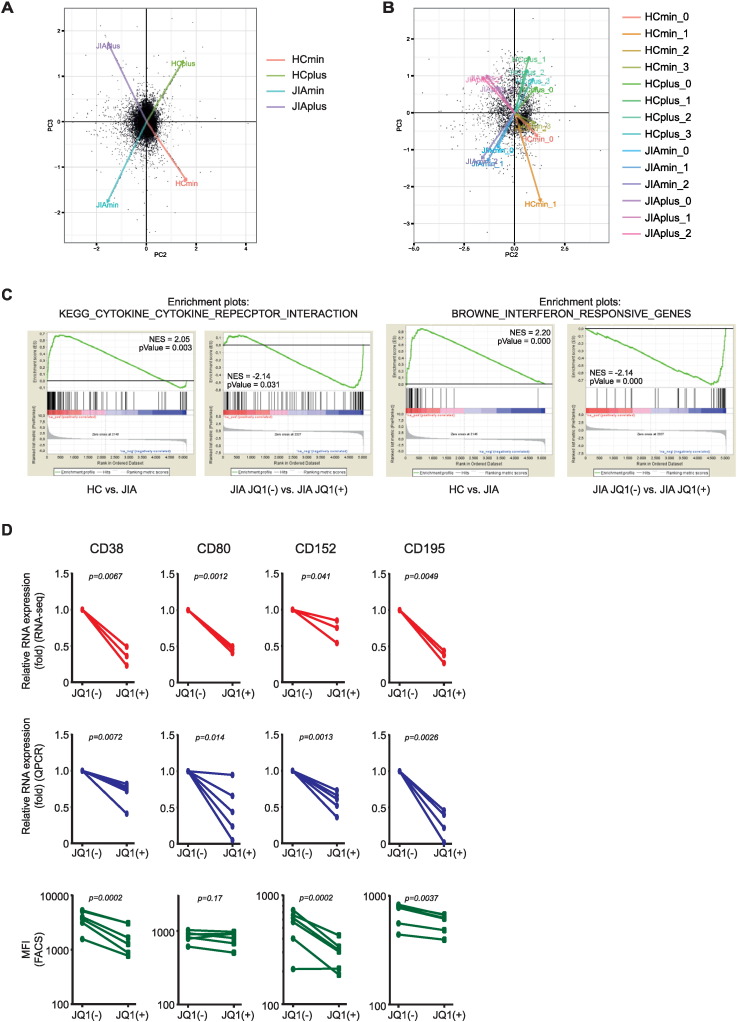
Principle component analysis (PCA) on the complete transcriptome of HC and JIA patient-derived T cells, either with and without JQ1(+) treatment, demonstrated that four different groups can be distinguished, illustrating that HC and JIA cluster separately and that JQ1 affects gene expression in such way that treated samples cluster separately from untreated samples (Fig. 2A).
Fig. 2.
Validation of RNA-sequencing data analysis. (A) Principal component analysis (PCA) performed on whole transcriptome of HC and JIA patient-derived CD4+CD45RO+ T cells treated with either JQ1(−) (HCmin and JIAmin) or JQ1(+) (HCplus and JIAplus). (B) PCA performed on significantly different genes between all replicates within the four groups analyzed. (C) Gene set enrichment analysis (GSEA) on ranked genes differentially expressed between either HC-derived Tmem/eff cells vs. JIA patient-derived Tmem/eff cells and JIA patient-derived Tmem/eff cells treated with JQ1(−) vs. JQ1(+) treatment. P value was determined using the familywise error rate procedure. NES = nuclear enrichment score. (D) Relative mRNA expression of CD38, CD80, CD152, and CD195 in JIA patient-derived Tmem/eff cells upon JQ1(+) treatment was measured by RNA-sequencing and qPCR. In addition, using flow cytometry, the mean fluorescence intensity of each CD-marker was assessed upon JQ1(+) treatment.
In addition, we performed PCA on the genes significantly expressed between all different groups, which demonstrated that all replicates cluster within the same group (Fig. 2B). Initial gene ontology term analysis revealed that the group of significantly genes upregulated in JIA compared to HC as well as the genes downregulated upon JQ1(+) treatment in JIA, correlated with a defense response. Additional gene set enrichment analysis (GSEA, Broad Institute) for genes differentially expressed between HC and JIA showed that the genes positively correlated with this comparison, i.e. genes that are upregulated in JIA, are enriched for the same gene subsets as the genes that are negatively correlated with the comparison JQ1(−) versus JQ1(+) treatment of JIA patient-derived T cells, i.e. genes that are downregulated by JQ1 (Fig. 2C). This again illustrates that genes affected by JQ1(+) strongly overlap with the genes that are upregulated in JIA. In addition to functionally grouped network analysis, GSEA also shows that genes associated with cytokines/cytokine receptor interactions are enriched within this subset of genes. To further validate our RNA-sequencing results, we selected some inflammation-associated (activation) markers that were downregulated upon JQ1(+) treatment and measured gene expression by qPCR. This resulted in the confirmation of downregulation of several CD markers on the RNA level, of which CD38, CD152, and CD195 also showed downregulation on the protein level, measured by flow cytometry (Fig. 2D).
3. Conclusion
In order to increase insight into molecular mechanisms playing a role in autoimmune diseases, we performed genome-wide transcriptome profiling of autoinflammatory-site derived Tmem/eff cells obtained from JIA patients. We observed that the majority of genes differentially expressed between JIA patients and HC were upregulated in JIA patient-derived T cells, strongly suggesting that these genes might contribute to the disease. Furthermore, we treated patient-derived T cells with the BET-inhibitor JQ1 and observed a preferential inhibition of the genes that were upregulated in JIA patients, i.e. the disease-associated genes. RNA-sequencing results were confirmed by qPCR and both PCA and GSEA showed that JQ1 can dramatically alter the cellular gene expression profile. In conclusion, our data suggests that BET-inhibition might be a novel, powerful therapeutic strategy in the treatment of autoimmune diseases.
Acknowledgments
This work was supported by the Dutch Arthritis Foundation (14-3-201) and The Netherlands Organization for Scientific Research (NWO). S.J. Vervoort is supported by the Dutch Cancer Society (KWF; UU 2013-5801).
References
- 1.Peeters J.G.C., Vervoort S.J., Tan S.C., Mijnheer G., de Roock S., Vastert S.J. Inhibition of super-enhancer activity in autoinflammatory site-derived T cells reduces disease-associated gene expression. Cell Rep. 2015;12:1986–1996. doi: 10.1016/j.celrep.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell C., Pachter L., Salzberg S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L., Wang S., Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 5.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]