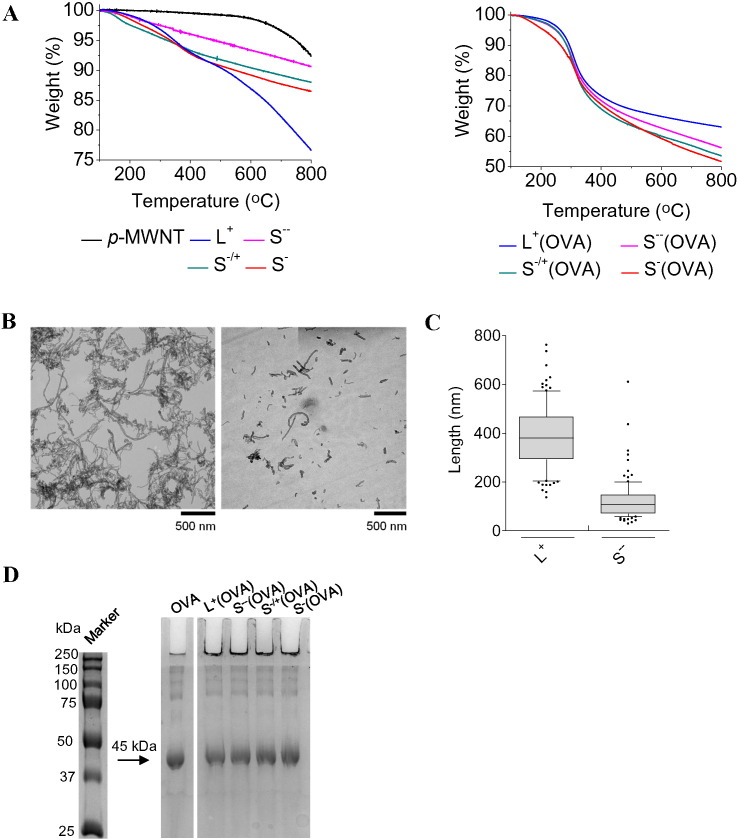
Fig. 1.
Physicochemical characterization of f-MWNTs and MWNTs-OVA. (A) Thermogravimetric profiles of f-MWNTs (left) or MWNTs-OVA (right). A known weight of MWNT was exposed to gradually increasing temperature and the weight loss was detected as the temperature increased. p-MWNTs were thermally stable up to 600 °C. The weight loss at 600 °C was directly correlated to the amount of introduced functional groups or OVA. Representative thermogravimetric profiles are shown (n = 3). (B) Morphology of f-MWNTs. Representative TEM images of L+ (left) and S−− (right), deposited on carbon grid from aqueous dispersions. S−− displayed shorter lengths compared to L+. (C) Box plot of L+ or S−− length distribution. The horizontal line inside the box indicates the median value; the black dots indicate values outside the 10–90 percentiles. Measurements were carried out on 100 individualized nanotubes and analyzed using ImageJ software. (D) Polyacrylamide gel electrophoresis of MWNTs-OVA. MWNTs-OVA were gel electrophoresed using 10% polyacrylamide gel under native gel condition. 10 μg of free OVA or OVA conjugated with MWNTs were loaded in the well. OVA bands were detected by gel staining with brilliant-Coomassie blue. Matching band intensities were observed for both the free OVA and MWNT-conjugated OVA.