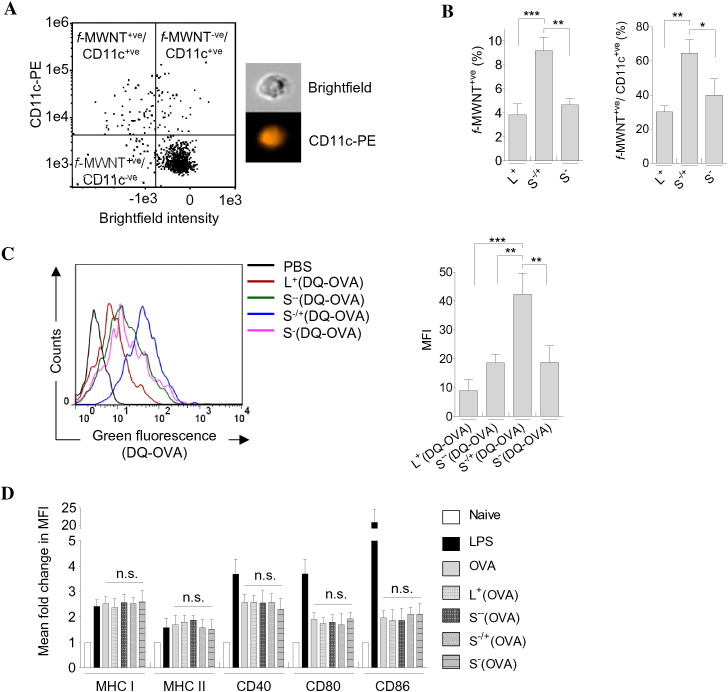
Fig. 5.
In vivo uptake and phenotypic characterization. (A) Uptake of f-MWNTs in draining popliteal lymph nodes. C57BL/6 mice (n = 3) were injected via the footpad with 100 μg of f-MWNTs, and the draining popliteal lymph nodes were dissected 24 h later. The isolated lymph node cells were stained for DCs using PE anti-CD11c (PE-CD11c) and analyzed using ImageStream analysis. Scatter plot of lymph node cells isolated from S−/+ injected mouse is shown, as a representative plot, illustrating the gating strategy applied to determine the f-MWNT+ ve and CD11c+ ve cells based on the reduction in bright-field intensity and the increase in PE-CD11c fluorescence intensity, respectively (cell images are shown in an inset). (B) Quantification of f-MWNTs uptake in popliteal lymph nodes. (Left) Percentage of f-MWNT+ ve cells in the whole cell population. (Right) Percentage of f-MWNT+ ve cells in the CD11c+ ve cells. (C) Uptake and processing of f-MWNT conjugated OVA in draining popliteal lymph nodes. C57BL/6 mice (n = 3) were injected with MWNTs(DQ-OVA), each contained 10 μg DQ-OVA. The isolated lymph node cells were stained with PE-CD11c and analyzed using flow cytometry. (Left) Representative histograms showing the processed DQ-OVA fluorescence, determined using the FL-1 detector. (Right) The MFI of processed DQ-OVA. (D) Effect of MWNTs-OVA on CD11c+ ve lymph node cells phenotypes. C57BL/6 mice (n = 2) were injected with OVA or MWNTs-OVA, each at 50 μg OVA. The isolated lymph node cells were stained with fluorescently-labeled antibodies and analyzed using flow cytometry. The MFI of the positive cells was determined to measure the fold change in the MFI of each marker compared to the naive cells. Results are expressed as mean ± S.D. Statistical analyses were performed using one-way ANOVA with Bonferroni post-test.