Figure 4. Phosphorylation of hAha1-Y233 Impacts the Chaperoning of Hsp90 Clients.
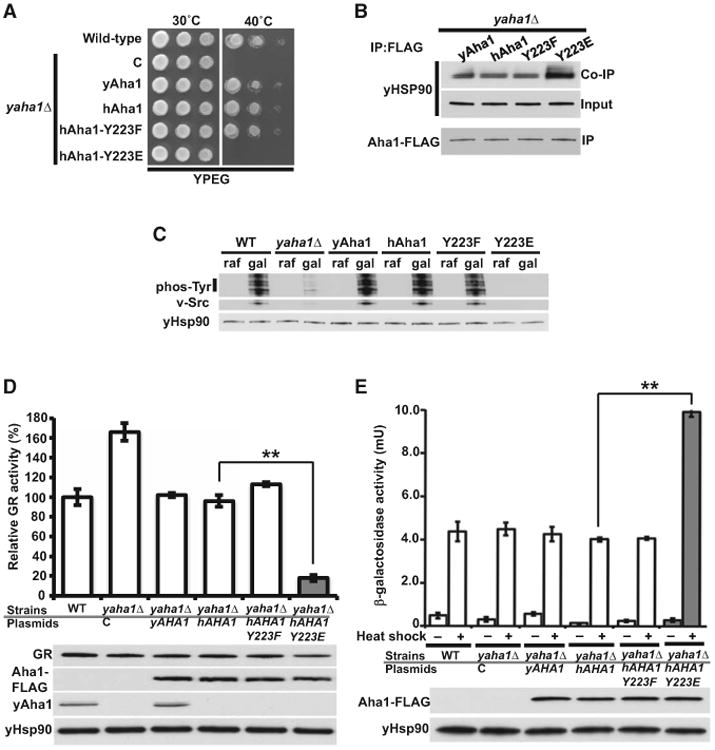
(A) yAha1 deleted cells (yaha1Δ) expressing empty-plasmid pRS314 (C), yAha1, hAha1, hAha1-Y223F, or hAha1-Y223E mutants were grown on YPEG (respiratory) liquid media at 28°C for 24 hr and 1:10 dilution series were spotted on YPEG agar. Plates were incubated at either 30°C or 40°C for 5 days.
(B) Yeast cells in (A) expressing indicated Aha1-FLAG under yAHA1 native promoter were used to immunoprecipitate (IP) Aha1 with anti-FLAG agarose. The co-immunoprecipitation (co-IP) of yHsp90 was analyzed by immunoblotting.
(C) Indicated yeast cells containing v-SRC under GAL1 promoter were grown on raffinose (–) or galactose (+) media. v-Src and total phosphotyrosine were analyzed by immunoblotting.
(D) GR-lacZ activity was assessed in the indicated yeast strains. The data are expressed as a percentage of the activity observed in WT cells. The mean ± SD from values obtained in three independent experiments with **p < 0.005 are presented. The levels of yAha1, hAha1-FLAG, yHsp90, and GR were analyzed by immunoblotting.
(E) Yeast strains in (A) with HSE-lacZ were heat shocked (40 min at 39°C). The heat shock response activity was measured in three independent experiments. The hAha1-FLAG and yHsp90 protein levels were assessed by immunoblotting. All the data represent mean ± SD (**p <0.005).
