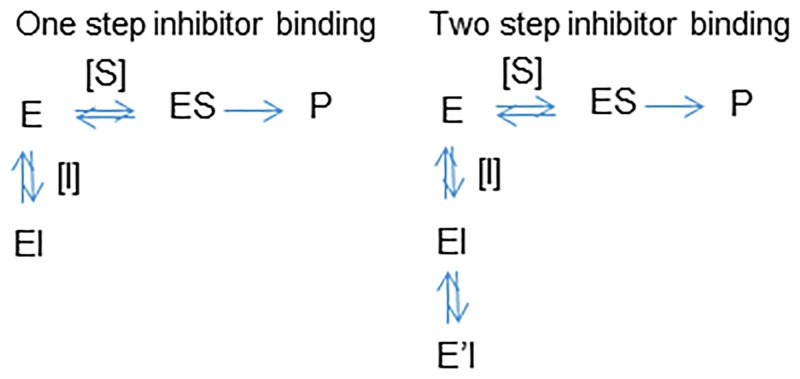
Fig 1. Kinetic scheme for slow binding kinetics.
One-step binding: the inhibitor [I] and substrate [S] are in equilibrium; binding is competitive and mutually exclusive. Increasing [S] will shift the equilibrium to product formation and reduce the amount of enzyme in bound to inhibitor (EI); for two-step binding the formation of the E’I complex is time dependent and not in rapid equilibrium with ES; increasing [S] will have a smaller effect on reducing the fraction of enzyme in the E’I complex. This behavior looks noncompetitive and is termed insurmountable.