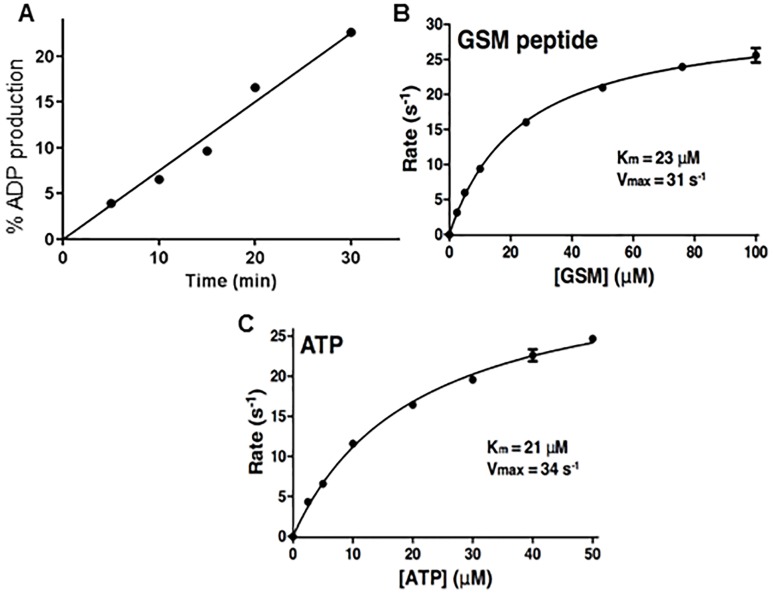
Fig 2. Substrate kinetics for TBGSK3β.
Panel A. Time course of TbGSK3β, 1.5 nM, run in kinase buffer (15 mM HEPES, pH 7.4, 10 mM MgCl2, 1 mM EGTA, 0.02% Tween 20) with 10 μM ATP and 10 μM GSM. Reactions were initiated with the addition of enzyme to a total volume of 20 μL. Microtubes were mixed and sealed, and reactions were terminated by placement of the tube in a heat block for 5 min. Reaction mixtures were transferred to a 384-well plate, and ADP-Glo (Promega) detection was used according to the manufacturer’s instructions. Panels B and C. Michaelis Menten plots of reaction velocity with increasing concentrations of (B) GSM peptide and (C) ATP. They velocities are calculated from the nmol of ADP formed/nmol of enzyme (TbGSK3β)/ time.