Background:
Although cleft lip and cleft palate are among the most common congenital malformations, the presence of an isolated congenital palatal fistula along with a submucous cleft is very rare. This appears as an oval-shaped, full-thickness fenestration in the palatal midline that does not fully extend anteriorly or posteriorly, accompanied by the findings of a submucous cleft. Because of the uncommon nature of this entity, there is controversy about its etiology, diagnosis, and management.
Methods:
Two cases of children with congenital palatal fistulae and a submucous cleft palate are presented who were treated in different settings by different surgeons. Cases are discussed along with a thorough review of the available literature.
Results:
Patient 1 presented at 4 years of age with “a hole in the palate” since birth and abnormal speech. His palatal fistula and submucous cleft were repaired with a modified von Langenbeck technique in Ethiopia. At a 2-year follow-up, the palate remained closed, but hypernasal speech persisted. Patient 2 was a 1-year-old presenting with failure to thrive and nasal regurgitation, who underwent a Furlow palatoplasty in the United States with good immediate results. She was unfortunately lost to follow-up.
Conclusions:
A congenital fenestration of the palate is rare. Reports reveal suboptimal speech at follow-up, despite various types of repair, especially when combined with a submucous cleft. Available literature suggests that repair should not focus on fistula closure only but instead on providing adequate palate length to provide good velopharyngeal function, as in any cleft palate repair.
Although clefts of the lip and palate are among the most common congenital malformations, the presence of an isolated congenital palatal fistula is rare. In 1904, when referring to phenotypic variations of clefts, von Bergmann noted that the rarest form was a cleft between the palatine processes of the maxilla only, with intact structures posteriorly and anteriorly.1 Subsequently, in 1931, Veau and Borel2 reported the first case of a congenital fistula of the hard palate.
Over the last century, there have been fewer than 30 cases described in the literature.1 Although it is difficult to assess the occurrence of such an uncommon condition, large cleft palate patient series estimate an incidence between 0.17% and 0.45% of all palatal clefts and 6% and 17% among those patients with a submucous cleft palate.3,4 Although it is not a rule, the congenital palatal fistula is commonly associated with a submucous cleft palate as described by Calnan’s triad: a posterior palatal notch, a zona pellucida due to muscle malposition, and a bifid uvula.
Because of varying ages at presentation and patient characteristics, etiology of this condition remains unclear. Regardless of the origin, there is little information in the literature that addresses the correction of this palatal defect. Reported correction techniques are highly variable, and most case reports have revealed suboptimal speech results at follow-up. This report presents 2 cases of congenital palatal fistulae across the globe, both in conjunction with a submucous cleft palate. Each case was corrected by a different surgeon using a different approach.
CASE 1
Burn/Cleft Lip and Palate Unit, Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia
A 4-year-old boy was sent to the Yekatit 12 Hospital Medical College in Addis Ababa, Ethiopia, by a local organization working with cleft lip and palate patients. His father reported that the child had nasal regurgitation of food and speech difficulties, and he had noticed a “hole” in his palate. There had been no history of trauma to the palate that he could recall. It was unclear whether this had been present since birth. Pregnancy had been uneventful, and the delivery had been at home with no complications. There was no family history of cleft lip and palate.
On examination, there was an 8 × 7 mm midline fistula at the junction of the hard and soft palates involving the posterior edge of the hard palate and creating a notch. This was accompanied by a submucous separation of the palatal muscles (Fig. 1A).
Fig. 1.
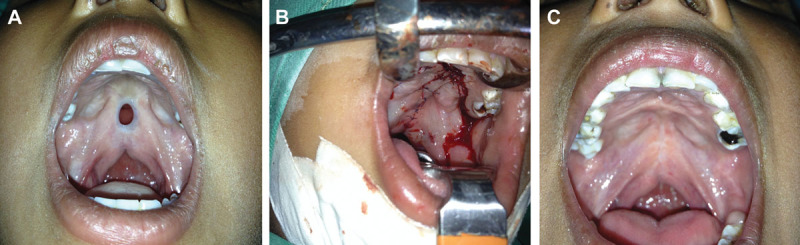
A, Congenital palatal fistula in case 1, at the junction of the hard and soft palates. Note the diastasis of the palatal muscles as seen in a submucous cleft palate and shortened velum. B, Palatal fistula repair using a modified von Langenbeck technique. See lateral incision that allows for midline closure. C, Two-year follow-up after von Langenbeck palatoplasty, showing full closure of the fistula.
Under general anesthesia, a modified von Langenbeck palatoplasty was performed. Lateral palate incisions allowed for closure of the fistula (Fig. 1B), and the levator veli palatini was released and repaired as a sling, in an attempt to improve speech. There were no complications postoperatively. A 2-year follow-up at 6 years of age showed a well-healed palate with no recurrence of the fistula (Fig. 1C). However, speech evaluation at that time revealed articulation and speech errors secondary to persistent mild hypernasality. Although speech therapy was recommended, the family could not stay in the city for treatment.
CASE 2
University of North Carolina Craniofacial Center, Chapel Hill, N.C.
A 1-year-old Hispanic girl was brought to clinic by her mother. She had a history of poor weight gain secondary to feeding problems and had been noted to have a fistula of the palate. She also suffered from recurrent chronic otitis media and frequent upper respiratory tract infections. The patient weighing 7 lb, 8 oz was born of an uncomplicated full-term gestation, and there was no history of trauma to the palate. The only family history related to the condition was a cleft palate in the child of a paternal first cousin.
On examination, the patient was normocephalic, with a mandible that was small for her size. There was a 4 × 8 mm midline palatal fistula at the junction of the hard and soft palates. She also had evidence of a submucous cleft palate, with aberrant insertion of the palatal muscles creating a zona pellucida and a bifid uvula. Nasal regurgitation was seen while feeding with a pigeon bottle, and snoring was observed during sleep.
Language was within normal limits according to the Receptive-Expressive Emergent Language Scale, fulfilled with information given by her mother and observation of the child in clinic. The Receptive-Expressive Emergent Language Scale is a validated checklist that uses observational information reported by parents or guardians to assess speech and language ability in infants and toddlers.
Magnetic resonance imaging (MRI) was performed to better appreciate the extent of the fistula. Fine 1-mm cuts were obtained through the mandible and maxilla to further elucidate the muscular architecture of her soft palate. Images revealed a subcentimeter (diameter) fistula at the junction of the hard and soft palates. Soft palatal musculature was present but did not cross the midline, with muscle fibers lying in a vertical orientation along the line of the submucous cleft that involved both the hard and the soft palates. Given the findings on MRI, repair was undertaken using the Furlow double-opposing Z-plasty technique.
A 3-layer closure was attempted, 1 nasal mucosal layer and 1 palatal muscle and 1 oral mucosal layer. Although complete closure of the nasal mucosal layer could not be obtained cephalad, full closure of the muscular and the oral mucosal layers was easily attained. The patient was discharged home on postoperative day 1, tolerating adequate oral intake. The patient was unfortunately lost to follow-up afterward.
DISCUSSION
Although cleft palate is one of the most common congenital malformations, an isolated congenital fistula of the hard palate is an extremely rare finding. Veau and Borel were the first to report the condition in 1931,2 and since then, there have only been about 30 cases published in the literature. Although these reported cases of a palatal fistula are all similar, there are certain differences that have led to controversy about the etiology of the condition.
There are different hypotheses regarding the etiology of a congenital palatal fistula. Controversy lies in whether the palatal fistula is the result of a true embryological malformation or is instead an acquired condition secondary to a ruptured mucosa in a submucous cleft palate, prenatally or postnatally. According to Veau when he first described it, the fistula resulted from the prenatal rupture of a submucous cleft palate, therefore, he called it a congenital entity. Other authors have agreed with this theory, supported by the concomitant submucous cleft palate associated to the fistula, and the fact that it is found early in the postnatal life or childhood.3–5 In other cases, intact submucous clefts have been recorded before appearance of a fistula or perforation, supporting the hypothesis of a spontaneous rupture or a traumatic etiology.5,6 However, Lynch et al7 and others have reported congenital palatal fistulas that are truly isolated, occurring in the absence of a submucous cleft palate.1,8 These still occurred in the same location and presented early in childhood without a history of trauma.
Overall, the majority of cases in the literature have been associated with a submucous cleft palate1,3,4 and have been present since birth. Different presentations appear to be the exception to an already rare entity. In cases in which the palatal fistula is associated with a submucous cleft palate, as in both our patients, clinical findings overlap with Calnan’s classic triad of a submucous cleft.9,10 First, the fistula usually occurs in the junction of the hard and the soft palates, which is also the area of maximal tension of the palate.3 The anterior edge of the fistula then extends into the hard palate, so that a posterior palatal notch can be palpated. The diastasis of the palatal muscles creates a zona pellucida where there is intact mucosa, posterior to the fistula. Lastly, there is usually a bifid uvula. Other findings of submucous cleft palate are usually present, such as shortening of the soft palate. This seems to be more dependent on the association with a submucous cleft palate rather than the fistula itself.
Ideally, these palatal fistulas should be discovered during a complete neonatal examination or in early infancy, as was the case in some studies.1,8,11 Some patients may be brought due to feeding issues, including nasal regurgitation and failure to thrive secondary to it, as was true of our second case (1 year old). Others may present later in childhood or as adults with speech abnormalities, such as nasal air emissions or hypernasal speech due to the fistula itself, worsened by a possibly inadequate velopharyngeal closure from the submucous cleft. This was the case in our first patient.
In the presence of less well-defined variants of submucous clefts or other atypical findings, additional studies may be of help. MRI can be performed to better characterize anatomy and muscle position around the fistula to plan for the type of repair.12 Additionally, speech evaluation by a speech pathologist should be an adjunct to surgical care to better assess VPI preoperatively and postoperatively, especially when there is overlap of a palatal fistula with the spectrum of a submucous cleft.
Although treatment is usually surgical, nonsurgical interventions exist, such as the fitting of an obturator. This alternative can be considered in scenarios where surgery is refused or where it is not an option for social reasons. For example, Karacan et al8 reported the spontaneous closure of an isolated palatal fistula in the absence of a submucous cleft palate, 16 months after parents denied treatment. In children who are acquiring speech, treatment should be more aggressive to avoid a negative impact.
In 1971, Fára4 published a case series of 4 patients with a palatal fistula and a submucous cleft. He noted that the surgical treatment of the fistula was simple, but satisfactory velopharyngeal competence was not easily achieved. Our first patient had persistent speech problems after surgical correction, perhaps in part because the technique did not provide enough velum length; due to the older age at presentation (4 years), he had acquired speech errors. This could have been treated with speech therapy, which unfortunately the family declined due to social and geographic reasons.
Although not all submucous clefts should be treated, as a large percentage are asymptomatic,13 the presentation with a concurrent fistula denotes a functionally different entity. Separation between the oral and nasal cavities should be achieved. There are as many reported techniques as there are surgeries to correct submucous clefts. Although there is not a consensus for this specific condition, the aims of treatment are the same across many reports: closure of the fistula, rearrangement of the palatal muscles, and lengthening of the short velum.3,4
Virtually any technique for correction of a submucous cleft palate that includes medial tissue reapproximation should obtain a fistula closure.8,14 However, the main concern must be lengthening of the soft palate and appropriate reconstruction of the palatal muscles, in a way that restores velopharyngeal function and prevents speech abnormalities. The von Langenbeck palatoplasty with intravelar veloplasty and Furlow double-opposing Z-plasty seem to be the most commonly used procedures for the correction of this condition in reports from the last few decades.8,13–15 These techniques are widely used for overt cleft palate repair and VPI correction and have shown good long-term speech outcomes. Regarding the von Langenbeck procedure, as used in our first case, Cheng and Zhou3 found no improvement in speech or velopharyngeal competence assessed radiologically and endoscopically. Our first patient was found to have persistent speech problems after 2 years, although it is unclear whether this was due to the technique itself or due to mislearning and lack of speech therapy. In contrast, our second patient had a Furlow palatoplasty performed before speech acquisition, but she was lost to follow-up. Unfortunately, most other published reports lack long-term follow-ups with speech assessment as well. From the available data, it seems that more than fistula closure alone, the treatment should focus on repairing aberrant musculature and lengthening the palate to provide good velopharyngeal function for correct speech, as in any cleft repair.3
SUMMARY
A congenital palatal fistula is a rare entity. In most published cases thus far, it has been associated with a submucous cleft. Although there is controversy regarding the exact etiology, a thorough understanding of the clinical problem should suffice to guide the appropriate treatment. If there is doubt after physical examination, MRI could be used as an adjunct to better characterize palatal musculature around the fistula for use in the repair. With that information, one can accurately decide which technique is the most appropriate for the tissue available. Although more data are available with long-term outcomes for this specific subset of patients, surgical treatment should be performed with the same main goals in mind as for any submucous cleft requiring surgical treatment, in terms of velar function and speech.
ACKNOWLEDGMENTS
We acknowledge Smile Train for supporting free cleft surgery for the patients in this study and other patients, Project Harar for bringing these patient to our center for cleft care, Transforming Faces for supporting holistic care of cleft patients, and Laura Lewis-Watts, Program Manager of Transforming Faces, for her help in finding and reviewing the relevant literature.
Footnotes
Disclosure: This project was supported, in part, by NIDCR grant R00 DE022378 (Dr. Butali); subaward to Addis Ababa University (Dr. Eshete). The other authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the corresponding author, Dr. Alexander Spiess.
REFERENCES
- 1.Rogers GF, Murthy A, Mulliken JB. Congenital fenestration of the palate: a case of embryologic syzygy. Cleft Palate Craniofac J. 2006;43:363–366. doi: 10.1597/05-013.1. [DOI] [PubMed] [Google Scholar]
- 2.Veau V, Borel S. Division Palatine: Anatomie, Chirurgie Phonétique. Masson: 1931. [Google Scholar]
- 3.Cheng N, Zhou M. Congenital fistula of the palate. J Craniomaxillofac Surg. 1998;26:391–393. doi: 10.1016/s1010-5182(98)80073-5. [DOI] [PubMed] [Google Scholar]
- 4.Fára M. Congenital defects in the hard palate. Observation of five cases. Plast Reconstr Surg. 1971;48:44–47. [PubMed] [Google Scholar]
- 5.Mehendale FV, Sommerlad BC. Submucous cleft palates presenting with a perforation. Cleft Palate Craniofac J. 2003;40:203–206. doi: 10.1597/1545-1569_2003_040_0203_scppwa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 6.Trelat U. Imperfect intonation and its causes. Lancet. 1870;96:553–554. [Google Scholar]
- 7.Lynch JB, Lewis SR, Blocker TG., Jr Cleft palate not explained by embryology. Plast Reconstr Surg. 1966;38:552–554. doi: 10.1097/00006534-196638060-00010. [DOI] [PubMed] [Google Scholar]
- 8.Karacan M, Olgun H, Tan O, et al. Isolated congenital palatal fistula without submucous cleft palate. J Craniofac Surg. 2009;20:1606–1607. doi: 10.1097/SCS.0b013e3181b14722. [DOI] [PubMed] [Google Scholar]
- 9.Calnan J. Submucous cleft palate. Br J Plast Surg. 1954;6:264–282. doi: 10.1016/s0007-1226(53)80060-3. [DOI] [PubMed] [Google Scholar]
- 10.Smiley GR. A possible genesis for cleft palate formation. Plast Reconstr Surg. 1972;50:390–394. doi: 10.1097/00006534-197210000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Tosun Z, Ozkan A, Karaçor Z, et al. Palatal perforation as a result of neonatal sepsis. Plast Reconstr Surg. 2005;116:1821–1822. doi: 10.1097/01.prs.0000188856.20267.2b. [DOI] [PubMed] [Google Scholar]
- 12.Kuehn DP, Ettema SL, Goldwasser MS, et al. Magnetic resonance imaging in the evaluation of occult submucous cleft palate. Cleft Palate Craniofac J. 2001;38:421–431. doi: 10.1597/1545-1569_2001_038_0421_mriite_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 13.Gosain AK, Hettinger PC. Submucous cleft palate. In: Losee JE, Kirschner RE, editors. Comprehensive Cleft Care. 1st ed. The McGraw-Hill Companies; 2009. pp. 361–370. [Google Scholar]
- 14.Shah S, Garg R, Uppal SK, et al. Sub mucous cleft palate with fenestration. Int J Appl Basic Med Res. 2014;4(suppl 1):S56–S57. doi: 10.4103/2229-516X.140745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aziz M. Congenital palatal fistula in a patient with submucous cleft palate. J Plast Reconstr Aesthet Surg. 2009;62:e509–e510. doi: 10.1016/j.bjps.2008.06.059. [DOI] [PubMed] [Google Scholar]


