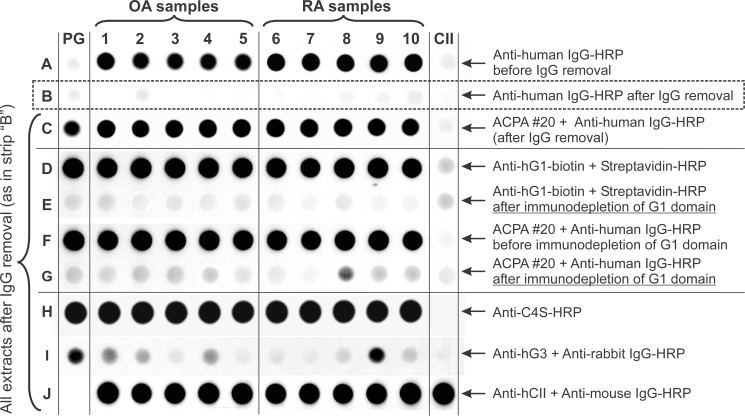
Fig 2. Recognition of PG in crude extracts of osteoarthritis (OA) and rheumatoid arthritis (RA) cartilage specimens by ACPA and PG-specific antibodies.
Dots of crude extracts of cartilage from OA donors (row A, samples 1–5) and RA donors (samples 6–10) were applied to a nitrocellulose membrane strip. Purified human PG and CII (far left and far right dots, respectively) served as controls. Blotting with the secondary antibody (anti-human IgG-HRP) revealed positive reactions (row A) with all crude extracts, but not with purified hPG or hCII. The “positive” reaction disappeared after the contaminating IgG was removed from the crude extracts (row B). These IgG-free cartilage extracts were used for subsequent dot blots. The ACPA+#20 serum (row A) reacted with all cartilage extracts and purified PG (row C). Similarly, the G1 domain-specific mAb recognized purified PG and PG in the crude extracts (row D), but no reaction was detected when the G1 domain was removed by immune absorption (row E). The reactivity of the cartilage extracts with ACPA+#20 serum (row F) was nearly completely lost after G1 domain immunodepletion (row G). As demonstrated by the anti-chondroitin 4-sulfate (C4S)-specific antibody, PG in all extracts and in the purified PG sample were glycosylated (row H). Some extracts and the purified PG contained small amounts of the PG G3 domain (row I), and all crude extracts contained cartilage-specific CII (row J).