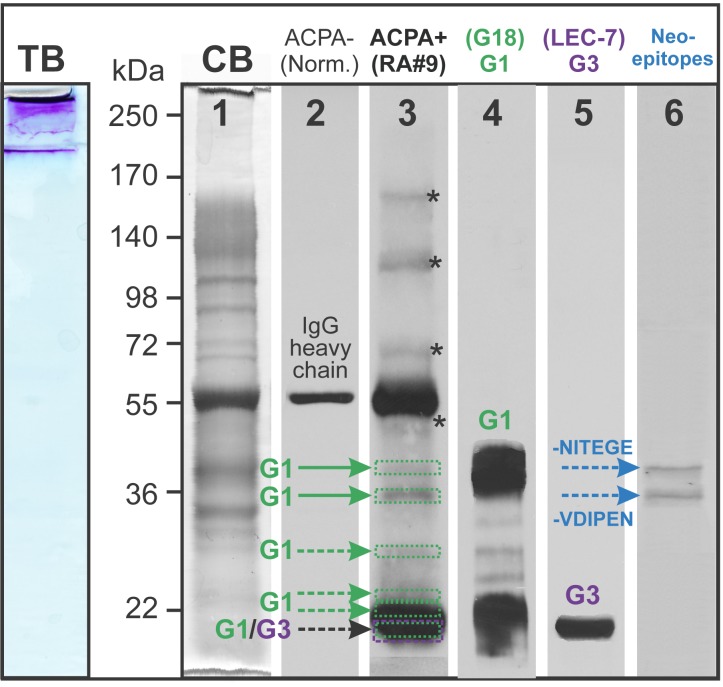
Fig 3. Western blots to identify PG domains and fragments of OA cartilage extract recognized by ACPA-positive serum.
The crude extract of OA cartilage (shown as sample 4 in Fig 2) was loaded onto 8% SGS-PAGE gel and stained with toluidine blue (TB) to visualize PG GAG chains. Essentially all of the TB-positive PG material remained in the stacking gel. To facilitate resolution, chondroitin sulfate chains were removed by digestion with chondroitinase ABC, and aliquots of the deglycosylated extract was loaded onto 6 lanes of a SDS-PAGE gel. Coomassie blue (CB) staining of the gel (lane 1) showed good resolution of the proteins of the OA cartilage extract after deglycosylation. Following transfer onto a nitrocellulose membrane, vertical strips of the membrane were probed with human sera or PG-specific antibodies (lanes 2–6). Immunostaining with ACPA- (normal) serum followed by anti-human IgG-HRP revealed a single protein band most likely corresponding to the heavy chain of contaminating IgG (lane 2). The ACPA+ serum detected several additional bands (lane 3). To identify these bands, replicate strips of the membrane were probed with antibodies against the G1 or G3 domain of PG and a pair of antibodies recognizing protease-generated PG neoepitopes. The respective antibodies showed reactions with the G1 (lane 4), G3 (lane 5) as well as with the neoepitopes -NITEGE and -VDIPEN (lane 6). There were additional bands above 55 kDa (depicted with asterisks in lane 3) that could not be identified as PG fragments. One representative sample of over 10 Western blots (using different crude extracts and ACPA+ sera) is shown.