Abstract
Xuefu Zhuyu Decoction (XFZY). a traditional Chinese medication and the biomedical medicine, vascular endothelia growth factor (VEGF), are both considered effective in treatment of ischemic cardiovascular disease because of their obvious angiogenesis function. VEGF may also cause plaque angiogenesis to accelerate atherosclerosis, while XFZY seems toresist atherosclerosis. The underlying mechanisms of their different angiogenesis behavior remain unknown. In this study, human microvascular endothelial cells (is “s” right) (HMEC-1) was (were?) employed to compare the angiogenesis cell behaviors of VEGF and XFZY treatment. VEGF promoted all cellular stages (or PHASES, your call) involved in angiogenesis including cell viability, proliferation, migration, adhesion and tube formation. Unlike the continuous promotion effects of VEGF on above stages, XFZY could inhibit cell viability and proliferation, and only promote tube formation in the early phase (“of angiogenesis”, your call). These results suggest that these two these two medications promote different angiogenesis behaviors which might be an important reason for their distinct therapeutic profile in clinical usage.
Keywords: XFZY, VEGF, Angiogenesis, Xuefu Zhuyu Decoction
1. Introduction
Vascular endothelial growth factor (VEGF) is an endothelial cells specific mitogen and the most widely used pro-angiogenesis factor used in ischemic research. Being the first approved? clinical drug for ischemic cardiovascular disease, VEGF may also hasten plaque angiogenesis to expand plaque which make it unstable and causes an acceleration of atherosclerosis. This angiogenesis activity at sites other than the target organ causes a Janus phenomenon (Epstein et al., 2004) whereby excessive angiogenesis may also increase potential risk of angiogenesis-borne diseases such as retinopathy and cancer (Masuda et al., 2008; Ohno-Matsui et al., 2002; Schwarz et al., 2000)
The herbal formula Xuefu Zhuyu (XFZY) Decoction, (“Drive out stasis from the mansion of blood”) was first recorded in 1830 AD in “Yilin Gaicuo” (“Corrections of Errors Among Physicians”), is a classic herbal formula for activating blood circulation and removing blood stasis. It is commonly used in traditional Chinese medicine to treat ischemic cardiovascular disease therapy. Instead of being a decoction, it is often prepared as a capsule in order to improve palatability, and increase the convenience dosing regime. Our preliminary works showed that XFZYD has pro-angiogenesis effect on chicken embryo chorioallantoic membrane (Gao et al., 2005), beside that, XFZYD could accelerate endothelial progenitor cells (Epcs) to form blood vessel in vitro (Gao et al., 2010b), mobilize Epcs to the ischemic region to repair the injure (Gao et al., 2012), and also induce endothelial cells (ECV304) migration, adhesion, proliferation and tube formation (Gao et al., 2010a). Clinical research showed that XFZYD is effective in anti-atherosclerosis and prevention of vascular restenosis after percutaneous transluminal coronary angioplasty (Shi et al., 1997; Yu et al., 1998). Unlike VEGF, XFZYD has not been implicated in any Janus phenomenon suggesting that XFZYD it may control angiogenesis without such risks. In this study we sought to investigate whether there are difference in the underlying mechanism of XFZY and VEGF in the treatment of ischemic cardiovascular. We employed HMEC-1 to compare the cell behaviors in angiogenesis induced by these two medications as a basis for the further mechanistic studies.
2. Materials and methods
2.1. Preparation of XFZY-CS
XFZY capsules was manufactured by Hongrentang Pharmaceutical Co., Ltd (batch No. A03039, Tianjin, China). Six male healthy volunteers were chosen approved by the institutional human experimentation committee. First, we drew 50ml venous blood for each person as the control serum. Then we gave participants 5 times the standard dose of XFZY capsules (equivalent to 2 times of crude dried herbs) per day. After 7 consecutive days, we drew venous blood again two hours after they ingested the capsules. Blood was centrifuged at 4000 r/min for 30 min and the XFZY-containing serum (XFZY-CS) was collected. Both the XFZY-CS and control serum were inactivated by heating at 56 °C for 30 min, sterile filtered through a 0.22 µm filter, and kept at −20 °C until use.
2.2. Cell Culture
HMEC-1 cell line was provided by Prof. Ding-jian from Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Cells were cultured in MCDB-131 (Sigma, USA) supplemented with 10% fetal bovine serum (FBS, GIBCO, USA), 10 ng/mL EGF (GIBCO, USA) and 1 µg/mL hydrocortisone. Cells in the logarithmic growth phase were selected and seeded in culture plates or culture flask. After adhered, they were starved in serum-free MCDB-131 for 12h and then randomly divided into VEGF groups and XFZY groups.
VEGF groups were stimulated with VEGF (Sigma, to make a series of final concentrations: 1.25 ng/mL, 2.5 ng/mL, 5 ng/mL and 10 ng/mL); XFZY groups were stimulated with XFZY-CS (to make a series of final concentrations: 1.25%, 2.5% and 5%). All groups were incubated in MCDB-131 supplemented with 5% FBS and treated with corresponding medicines or controls for 24h, 48h and 72h.
2.3. Cell Viability Assay
A total of 3×103cells/well were seeded into the 96-well plates. After treatment with the corresponding medication or controls, 20 µL methyl thiazolyl tetrazolium solution (MTT, 5 mg/ml) was added into each well. Following incubation for 4h, the supernatant was removed and HMEC-1 preparation was shaken with 150 µL dimethyl sulfoxide (DMSO) for 10 min before the optical density (OD) was assessed at 570 nm (reference wave, 630 nm) using a 96-well microplate reader (BioTek Co., USA).
2.4. Flow cytometry
Cells cycle was tested by FACS as described in the instruction book of Cycle TEST™ plus DNA REAGENT KIT (Becton Dickinson, USA). Cell Quest software (Becton Dickinson, USA) was used to acquire data, and ModiFit Version 3.0 (Becton Dickinson, USA) was used for analysis.
2.5. Cell migration assay
Cell migration was tested in Boyden Chambers (Jiangsu Qilin Medical Instrument Factory) with a polycarbonated filter (8 µm pore size, Whatman, Britain). A total of 5×104cells were plated into the upper chamber containing 100 µL of medium supplemented with 5% FBS. Lower chamber were filled with 150 µL of the corresponding cell culture supernatant. The cells were allowed to migrate for 8 hours. Following incubation at 37 °C, the cells on the upper side of the membrane were removed, the membrane was fixed with 4% neutral formalin for 20 min and cells were stained with hematoxylin. Photographs were taken at a magnification of 200× by an inverted phase contrast microscope (IX70, Olympus, Japan). The stained cells from 6 randomly selected fields were counted.
2.6. Cell adhesion assay
Cells were seeded in a 96-well plate coated with 1% gelatin (SIGMA, USA) and incubated for 60 min. then discarded the medium with non-adhesion cells, the adhesion cells on the plate were fixed with 4% neutral formalin for 20 min and stained with 0.1% crystal violet. The stained cells from 6 random fields (200×) were counted.
2.7. Endothelial cell tube formation assay
A ice-cold 96-well plate was coated with 50 µL/well Matrigel (Millipore Co., USA) which was allowed to solidify at 37 °C for 60 min followed the manufacturer’s instruction. HMEC-1 were seeded at a total of 1.8×105 cells in 150 µL/well culture medium supplemented with 5% FBS in triplicate. After 4h incubation in 37 °C, 5% CO2, images were taken for each well at a magnification of 200× using an inverted phase contrast microscope (IX70, Olympus, Japan). The number of tubes from 6 randomly selected fields were counted.
2.8. Statistical analysis
All data were expressed as mean±S.D. from three separate experiments. In order to avoid the different serum content effect, XFZY treated groups were compared with the same serum content control group using t-tests. VEGF treated groups were compared with the control group using one-way ANOVA, where significance was determined using LSD test. P<0.05 was considered as statistically significant.
3. Results
3.1. Effects of the two medicines on cell viability
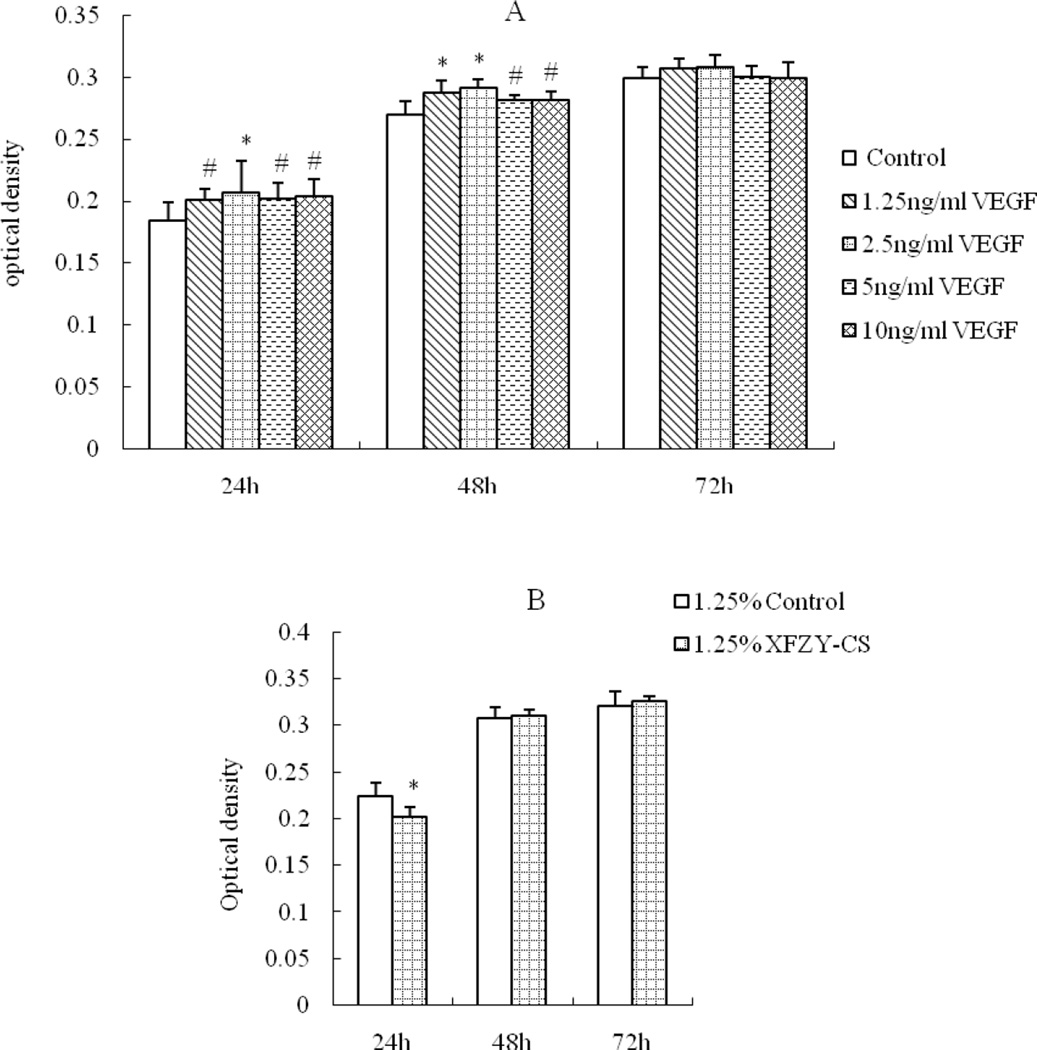
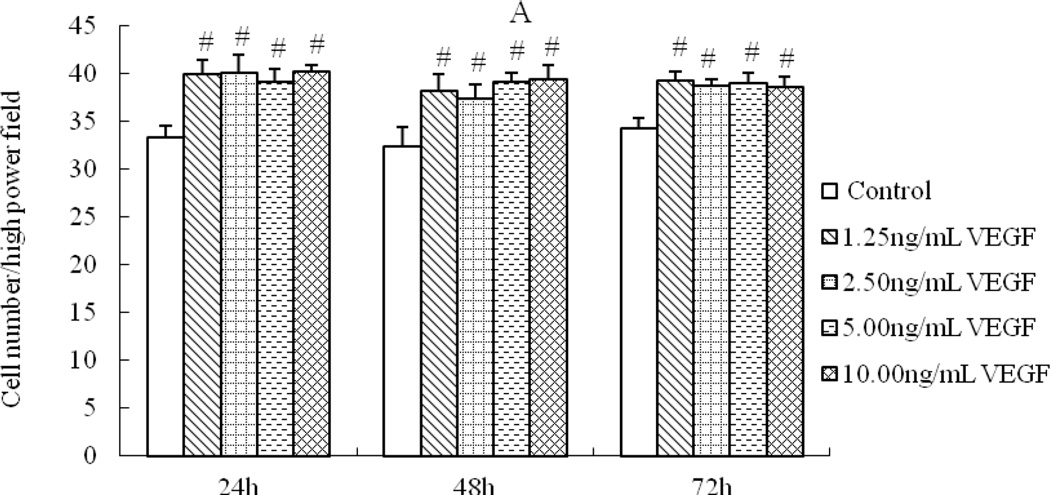
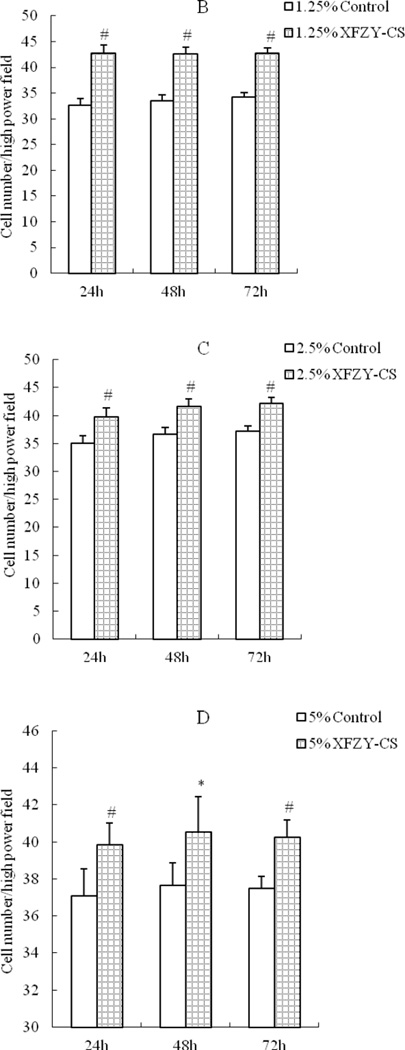
1.25 ng/mL VEGF was sufficient to enhance cell activity (Fig 1A), the effect was nor time-dependent or dose-dependent. While compared with the equivalent serum content control group, the 1.25% and 2.5% XFZY-CS inhibited cell activity at first 24h and the 5% XFZY-CS promoted cell activity at 48h, then all effects disappeared at 72h (Fig 1B). The results suggested that the promotion function of VEGF was earlier and longer than XFZY capsule on the HMEC-1 activity.
Fig 1.
Effects of VEGF and XFZY on cell viability. (A) VEGF promoted cell viability at 24h and 48h. (B and C) At 24h, both 1.25 and 2.5% XFZY-CS inhibited cell viability. (D) At 48h, 5% XFZY-CS promoted cell viability. *p<0.05 and #p<0.01 indicated statistical significance when compared with control.
3.2. Effects of the two medications on cell cycle
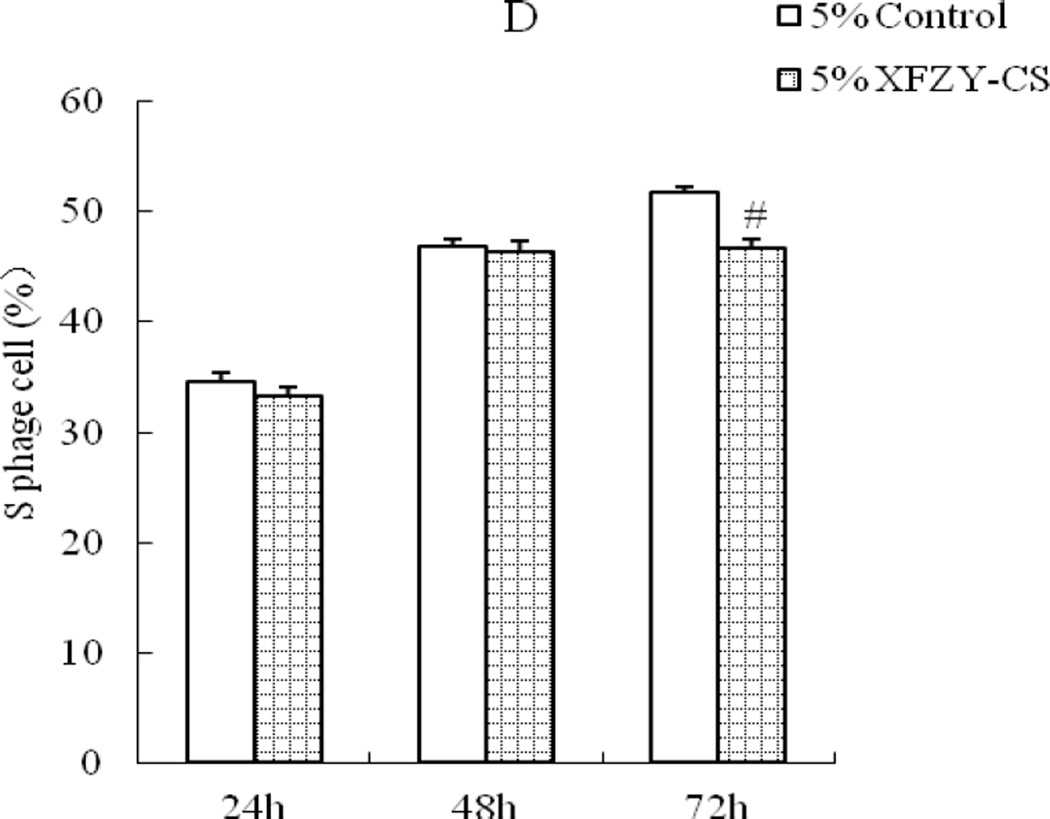
1.25 ng/mL VEGF was enough to make a time-dependent effect on raising the ratio of S phase cell (Fig 2A). While compared with the equivalent serum content control group, 2.5% and 5% XFZY-CS showed inhibition effect only at 72h (Fig 2B). The two medications exhibited opposite effect on cell proliferation.
Fig 2.
Effects of VEGF and XFZY on cell proliferation. (A) VEGF elevated the S phage cell ratio at 24h, 48h and 72h. (C and D) At 72h, 2.5% and 5% XFZY-CS decreased the S phage cell ratio. #p<0.01 indicated statistical significance when compared with control.
3.3. Effects of the two medications on cell migration
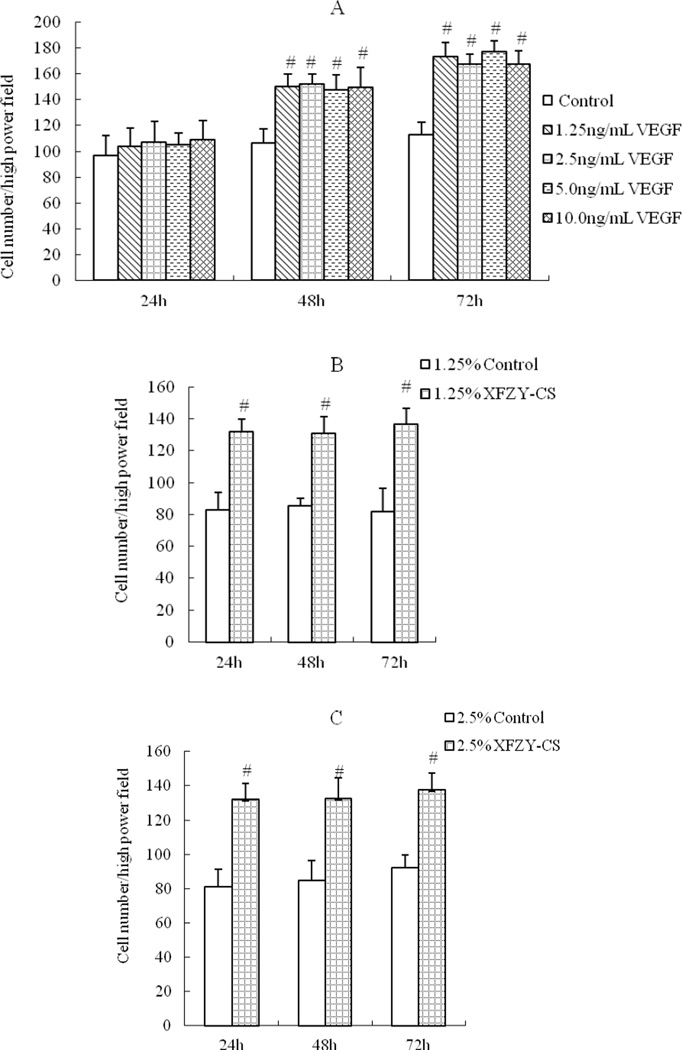
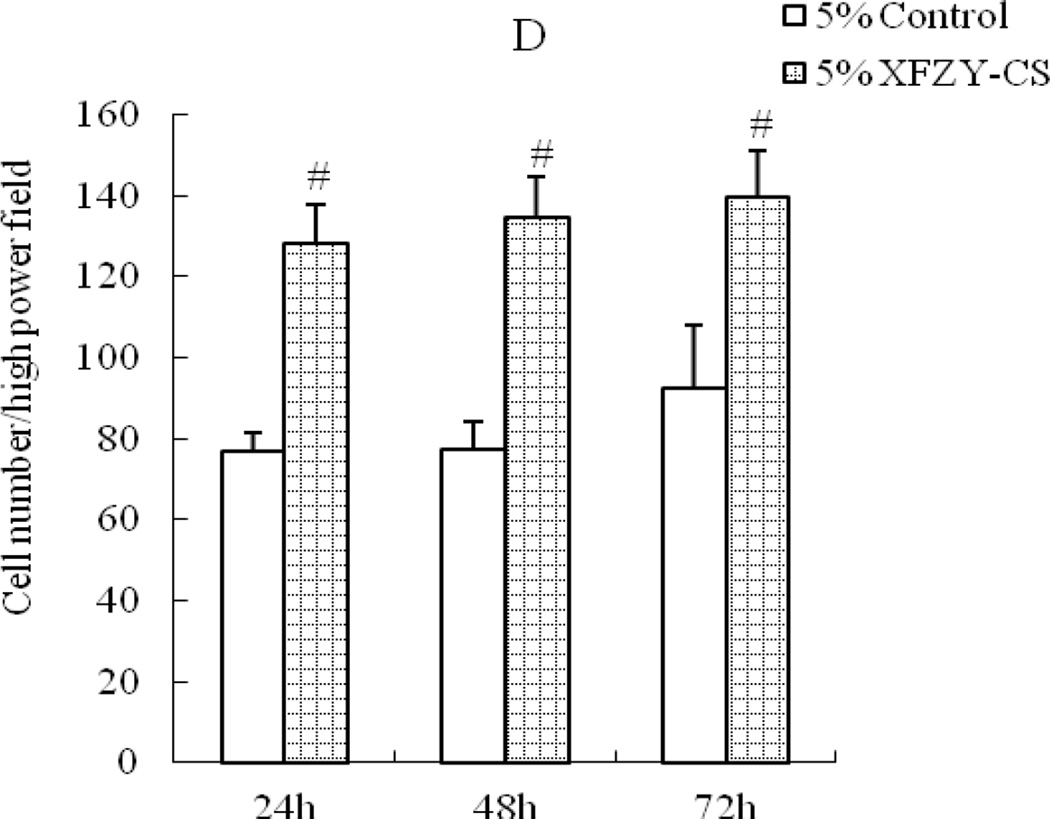
Considering that VEGF is? a chemotactic factor but XFZY is? not, cell culture supernatant was chosen to put in the lower chamber to showed the effect of medications working on the chemotactic factors. VEGF groups induced cell migration in a time-dependent manner (Fig 3A), no obvious? effect of VEGF at the first 24h, then an improvement of cell migration appeared at 48h and even stronger at 72h. While compared with the same serum content control group, all XFZY groups elevated cell migration as early as 24h (Fig 3B) which suggested a quicker onset for this cellular behavior than VEGF and this none time-dependent work lasted to 72h.
Fig 3.
Effects of VEGF and XFZY on cell migration. (A) VEGF increased cell migration at 48h and 72h. (B, C and D) 1.25, 2.5 and 5% XFZY increased cell migration at all 3 time points. #p<0.01 indicated statistical significance when compared with control.
3.4. Effects of the two medication on cell adhesion
The 1.25–10 ng/mL VEGF group elevated the cell number adherance to gelatin at the? three time points (Fig 4A). While compared with the equivalent serum content control group, all XFZY groups induced cell adhesion at 3 time points in a manner similar to VEGF (Fig 4B).
Fig 4.
Effects of VEGF and XFZY on cell Adhesion. (A) VEGF (1.25, 2.5, 5.0 and 10 ng/mL) promoted cell adhesion at all 3 time points, (B, C and D) 1.25, 2.5 and 5% XFZY increased cell adhesion at all 3 time points, respectively. *p<0.05 and #p<0.01 indicated statistical significance when compared with control.
3.5. Effects of the two medications on tube formation (I think don’t need capitals)
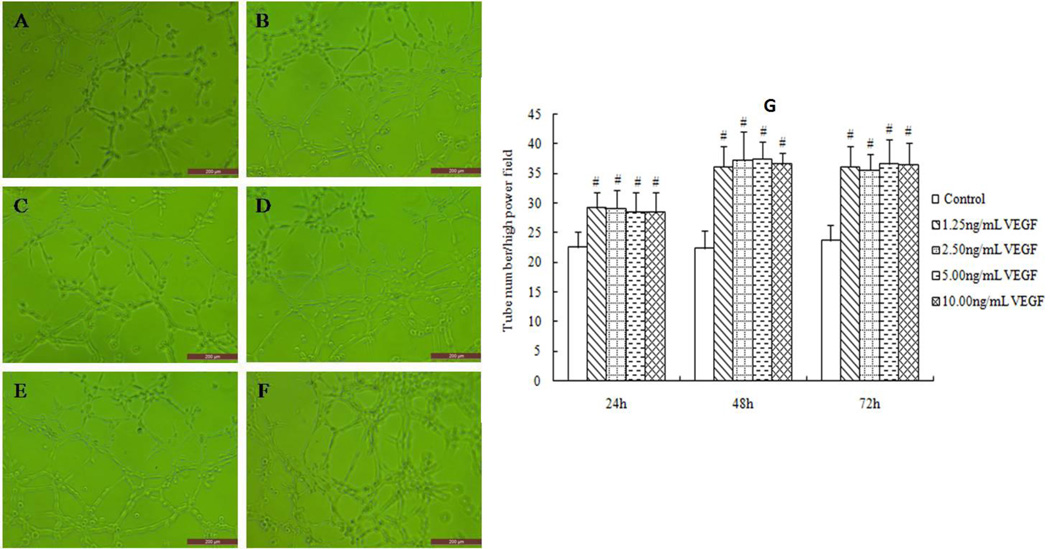
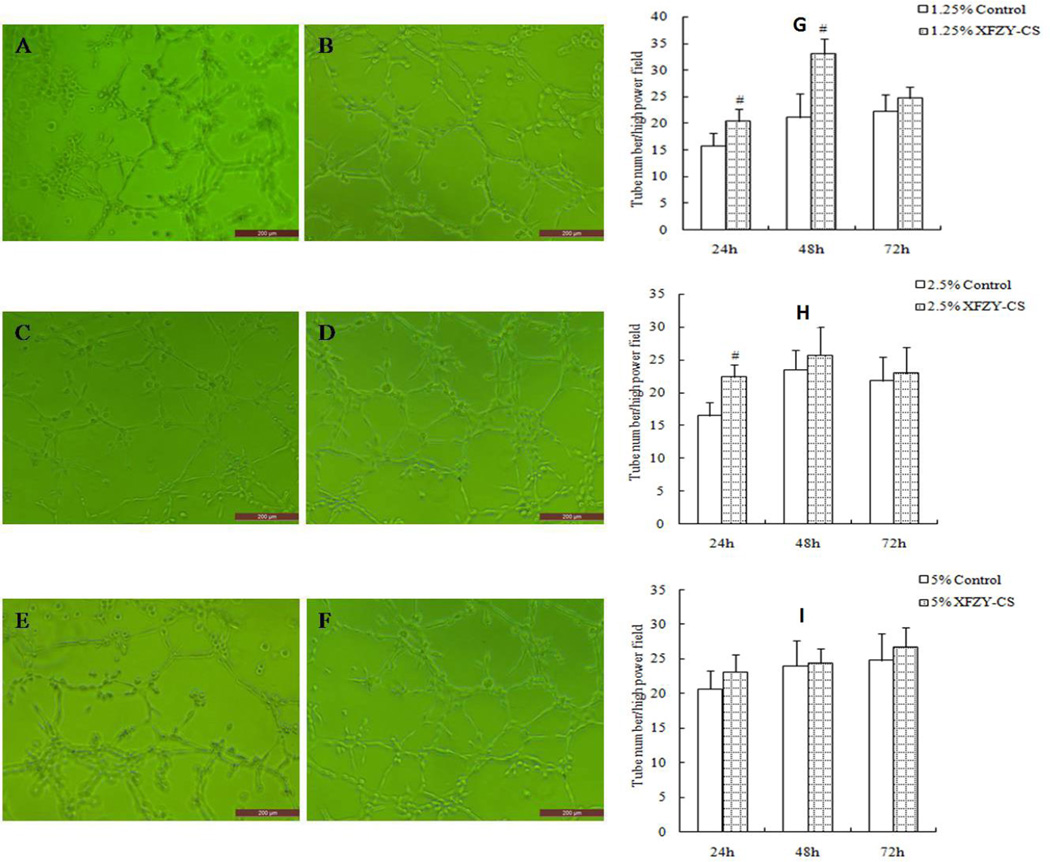
The VEGF groups promoted HMEC-1 tube formation at the? three time points (Fig 5A). While compared with the equivalent serum content control group, 1.25% XFZY group improved tube formation at 24 and 48 hours respectively (Fig 6B, 6D), and 2.5% XFZY worked only at 24h, then the pro-angiogenesis effect disappeared at 72h. The results suggest that low concentration of XFZY promoted? tube formation only during the early phase. This effect of XFZY did not have the persistence and sensitivity effects? of VEGF.
Fig 5.
VEGF promoted tube formation at all 3 time points. (A and B) At 24h, cells were cultured with VEGF-free medium (A) and 1.25 ng/mL VEGF (B). (C and D) At 48h, cells were cultured with VEGF-free medium (C) and 1.25 ng/mL VEGF (D). (E and F) At 72h, cells were cultured with VEGF-free medium (E) and 1.25 ng/mL VEGF (F). Scale bars: 200 µm. #p<0.01 indicated statistical significance when compared with control.
Fig 6.
XFZY promoted tube formation. (A and B) At 24h, cells were cultured with 1.25% control serum (A) and 1.25% XFZY. (C and D) At 48h, cells were cultured with 1.25% control serum (C) and 1.25% XFZY (D). (E and F) At 72h, cells were cultured with 1.25% control serum (E) and 1.25% XFZY (F). #p < 0.01, compared with equivalent serum content of control serum, t-test. Scale bars: 200 µm.
4. Discussion
As a functional factor in vivo, VEGF can be added directly into the cell culture medium. Because XFZY capsules consists of eleven Chinese herbs and its active pharmacological ingredients in serum are still unknown, the serum pharmacological method was adopted to investigate the effect of XFZY on HMEC-1. XFZY ingredients in serum were treated as one compound to be compared with VEGF. Following the recommendations of Dr. Guo Shikui, a famous elder Chinese physician at Xiyuan Hospital, Beijing with extensive experience in cardiovascular disease, we used 2 times the standard dosage of XFZYD that is usually recommended to patients with severe angina pectoris or insufficient response to routine treatment.. This is equivalent dose to 5 times dosage of XFZY capsules by taking off the auxiliary material weight. We used healthy human volunteers and did not use serum from Different from experimental animals in order, in order to avoid species differences and imitate clinical reality more closely. <--this can also go into the methods maybe
Angiogenesis is a complication process involved with vascular endothelial cell migration, proliferation, adhesion and formation of new blood vessels. In this study, except for cell adhesion, the two medicines tested -– VEGF and XFZY--- worked in in a different manner on each of the phases. VEGF promoted promote all stages. In contrast, XFZY could inhibited cell vigor or proliferation in a certain concentration, and induced tube formation limited in the low concentration and only over a short time frame. This short early pro-angiogenesis phenomenon of XFZY also appeared in vivo in an earlier pilot study investigation of repairing rat ischemic hind limb impairment (Gao et al., 2012). XFZY could only elevated the vessel number in granulation tissue at the early phase of repair, whereas the vessel number? did not change in the ischemic muscle, and the new vessel would occlude with the granulation tissue fibrosis. These results suggested that XFZY may control angiogenese in a different manner from the continuously function of VEGF.
XFZY is not the only Chinses medicine that can control vessel growth. Shexiang Baoxin Pills. a medication for coronary heart disease appears to promote angiogenesis on the rat myocardial infarction model (Wang et al., 2004) but inhibit vessel growth in cancer (Zhang et al., 2007). These two findings suggest that controlling vessel growth may be a characteristic of Chinese medicine. This might also explain the low rate of adverse events with Chinese medicine as compared to the side effect of VEGF in clinical usage.
In some ways, XFZY action on angiogenesis resembles VEGF. Early work by our group showed that XFZYD could induce angiogenesis in endothelial progenitor cells (Gao et al., 2010c) and endothelial cell ECV304 via VEGF pathway (Gao et al., 2010a) and elevate serum VEGF level in rats (Gao et al., 2010d). Also, XFZYD can affect some other angiogenesis regulator such as NO, bFGF, etc (Gao et al., 2010a; Gao et al., 2011). This multi-target action of XFZY may be responsible for the different angiogenesis behavior XFZY has compared to VEGF. The mechanisms of action of XFZY still require further in-depth research to develop a comprehensive and clear understanding. Undoubtedly, the effective ingredients of XFZYD in serum and its action mechanism are worth of further investigation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NO. 81072933 and NO. 81173431). TJK was partially supported by NIH, NCCAM grant # 2K24 AT004095.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Reference
- Epstein SE, Stabile E, Kinnaird T, Lee CW, Clavijo L, Burnett MS. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation. 2004;109:2826–2831. doi: 10.1161/01.CIR.0000132468.82942.F5. [DOI] [PubMed] [Google Scholar]
- Gao D, Chen WY, Wu LY. Effect of xuefu zhuyu decoction in inducing angiogenesis gene regulation of endothelial cell line ECV304. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010a;30:153–156. [PubMed] [Google Scholar]
- Gao D, Chen WY, Wu LY, Zheng LP, Lin W, Song J, Chen KJ. Effect of Xuefu Zhuyu Decoction on the Nitric oxide in the Endothelial Angiogenesis. Journal of Traditional Chinese Medicine. 2011;52:1852–1855. [Google Scholar]
- Gao D, Jiao YH, Wu YM. Experimental study of Xuefu Zhuyu Decoction induced participation of endothelial progenitor cells in the angiogenesis of the ischemic region. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:224–228. [PubMed] [Google Scholar]
- Gao D, Song J, Hu J, Lin J, Zheng L, Cai J, Du J, Chen KJ. Angiogenesis promoting effects of Chinese herbal medicine for activating blood circulation to remove stasis on chick embryo chorio-allantoic membrane. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:912–915. [PubMed] [Google Scholar]
- Gao D, Wu LY, Jiao YH, Chen WY, Chen Y, Kaptchuk TJ, Lu B, Song J, Chen KJ. The effect of Xuefu Zhuyu Decoction on in vitro endothelial progenitor cell tube formation. Chin J Integr Med. 2010b;16:50–53. doi: 10.1007/s11655-010-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Wu LY, Jiao YH, Chen WY, Chen Y, Lu B, Song J, Chen KJ. Effect of VEGF-VEGFR Pathway on EPCs Function Induced by Xuefu Zhuyu Tang. Chinese Journal of Experimental Traditional Medical Formulae. 2010c;16:104–107. [Google Scholar]
- Gao D, Wu LY, Jiao YH, Chen WY, Zheng LP, Song J, Chen KJ. Influencing Factors of Xuefu Zhuyu Decoction in Mobilization of Endothelial Progenitor Cells in Bone Marrow. Journal of Traditional Chinese Medicine. 2010d;51:457–459. [Google Scholar]
- Masuda K, Teshima-Kondo S, Mukaijo M, Yamagishi N, Nishikawa Y, Nishida K, Kawai T, Rokutan K. A novel tumor-promoting function residing in the 5' non-coding region of vascular endothelial growth factor mRNA. PLoS Med. 2008;5:e94. doi: 10.1371/journal.pmed.0050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, Duh EJ, Hackett S, Chang M, Bok D, Zack DJ, Campochiaro PA. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, Kloner RA. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat--angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–1330. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- Shi D, Li J, Ma X, Zhang Q, Mao J, Chen M, Chen K. Clinical observation on prevention of restenosis in cases with coronary heart disease after percutaneous transluminal coronary angioplasty with concentrated Xuefu Zhuyu pill. Journal of Traditional Chinese Medicine. 1997;38:27–29. [Google Scholar]
- Wang D, Li Y, Fan W. Effect of decreasing infarct area and stimulating angiogenesis of Shexiangbaoxin Pills in rat with coronary occlusion. Chinese Traditional Patent Medicine. 2004;26:54–57. [Google Scholar]
- Yu B, Chen K, Mao J, Guo J, Lv S. Clinical study on effect of concentrated xuefu zhuyu pill on restenosis of 43 cases coronary heart disease after intracoronary stenting. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18:585–589. [PubMed] [Google Scholar]
- Zhang C, Cheng K, Guo W, Wei HC. Effects of Shexiang Baoxin pill on Preangiogenesis factors and tumor growth of human colon cancer liver metastasis in nude mice. Journal of Pharmaceutical Practice. 2007;25:295–296. 302. [Google Scholar]