Fig. 3.
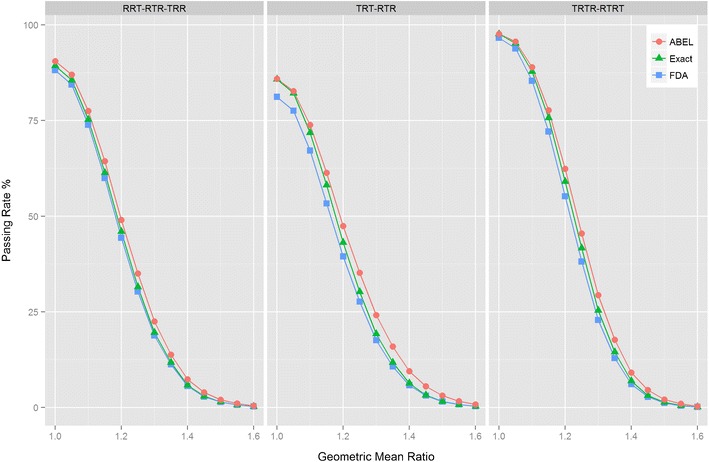
RSABE evaluated from simulated crossover bioequivalence studies with different study designs. The designs were as follows: four-period, two-sequence replicate design (RTRT-TRTR); three-period, two-sequence replicate design (TRT-RTR); and three-period, three-sequence partial replicate design (RRT-RTR-TRR). The dependence of the percentage of accepted BE studies is shown at various ratios of the geometric means (GMR) of the two formulations. It was assumed that the true within-subject standard deviations for both products were 0.4, and 24 subjects received the Test and Reference formulations in the simulated trials
