Abstract
Background
Deforestation and land-use change have the potential to alter human exposure to malaria. A large percentage of Madagascar’s original forest cover has been lost to slash-and-burn agriculture, and malaria is one of the top causes of mortality on the island. In this study, the influence of land-use on the distribution of Plasmodium vectors and concomitant Plasmodium infection in humans and mosquito vectors was examined in the southeastern rainforests of Madagascar.
Methods
From June to August 2013, health assessments were conducted on individuals living in sixty randomly selected households in six villages bordering Ranomafana National Park. Humans were screened for malaria using species-specific rapid diagnostic tests (RDTs), and surveyed about insecticide-treated bed net (ITN) usage. Concurrently, mosquitoes were captured in villages and associated forest and agricultural sites. All captured female Anopheline mosquitoes were screened for Plasmodium spp. using a circumsporozoite enzyme-linked immunosorbent assay (csELISA).
Results
Anopheles spp. dominated the mosquito communities of agricultural and village land-use sites, accounting for 41.4 and 31.4 % of mosquitoes captured respectively, whereas Anopheles spp. accounted for only 1.6 % of mosquitoes captured from forest sites. Interestingly, most Anopheles spp. (67.7 %) were captured in agricultural sites in close proximity to animal pens, and 90.8 % of Anopheles mosquitoes captured in agricultural sites were known vectors of malaria. Three Anopheline mosquitoes (0.7 %) were positive for malaria (Plasmodium vivax-210) and all positive mosquitoes were collected from agricultural or village land-use sites. Ten humans (3.7 %) tested were positive for P. falciparum, and 23.3 % of those surveyed reported never sleeping under ITNs.
Conclusions
This study presents the first report of malaria surveillance in humans and the environment in southeastern Madagascar. These findings suggest that even during the winter, malaria species are present in both humans and mosquitoes; with P. falciparum found in humans, and evidence of P. vivax-210 in mosquito vectors. The presence of P. vivax in resident vectors, but not humans may relate to the high incidence of humans lacking the Duffy protein. The majority of mosquito vectors were found in agricultural land-use sites, in particular near livestock pens. These findings have the potential to inform and improve targeted malaria control and prevention strategies in the region.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-016-1164-2) contains supplementary material, which is available to authorized users.
Keywords: Disturbance ecology, Ifanadiana District, Plasmodiumfalciparum, Plasmodiumvivax, Bed net
Background
Madagascar is a malaria-endemic country where malaria ranks fourth among causes of reported mortality. In 2011, malaria was the second leading cause of death among children under 5 years [1]. While malaria epidemiology varies considerably in different regions of the country, with perennial transmission in the southeastern regions of Madagascar [1], specific transmission seasons in the lowland areas in the northwest, and unstable seasonal transmission in the highland (central) and semi-desert areas (southwest) areas of the country, the entire population is considered to be at risk for the disease [1].
Of the five Plasmodium species capable of infecting humans with malaria, four are present in Madagascar [2], with Plasmodium falciparum as the most prevalent species, followed by P. vivax [2]. According to [2], the expected vector species of Anopheles in the region are Anopheles funestus, An. gambiae, and An. arabiensis, and more recently An. coustani [3, 4]. Although increased financing for malaria treatment and mosquito control with insecticides has successfully decreased malaria rates in Madagascar [1], addressing environmental determinants of disease could provide additional ways of controlling malaria, including environmental management practices, such as vegetation clearance, draining swamps and modification of river boundaries [5, 6], that would increase the efficiency of programmes [5].
Madagascar is a unique island environment with a high level of animal and plant endemism; however, to date over 90 % of the original forests on the island have been lost due to slash-and-burn agricultural practices associated with human population growth, which have led to rapid conversion of forest to land used for rice production [7]. In other parts of the world, this sort of deforestation has been linked to deleterious effects on environmental conditions capable of enhancing opportunities for human pathogens [8, 9]; and the loss of ecosystem resources through land-use conversion also has the potential to increase the risk of human malaria infection by altering nutrient enrichment and watershed dynamics on a local scale, creating an abundance of new Anopheles mosquito breeding habitats [8–11], for example, through irrigation, which often leads to an increase in mosquitoes and malaria prevalence [5].
In an effort to protect the original forests from land-use conversion, several national parks, such as Ranomafana National Park (RNP) in the southeastern rainforests of Madagascar, have been established [12]. Despite its protected status, RNP is threatened by accelerating forest conversion due to slash-and-burn agricultural practices. More than 85 % of the estimated 54,000 people in the region immediately surrounding RNP rely primarily on subsistence agriculture and the slash-and-burn practice remains a cultural norm [13]. Considering the importance of malaria as a cause of mortality in Madagascar [2], an understanding of the relationship between land-use conversion and malaria vector distribution and concomitant Plasmodium infection in humans and mosquito vectors on a local scale is necessary. Consequently, this study was conducted to: (1) determine the distribution of Plasmodium vectors and concomitant Plasmodium-status of vectors relative to small-scale variability in land-use patterns, and (2) examine demographic and behavioural associations with human Plasmodium spp. infection in this system.
Methods
Ethics statement
All research protocols were presented to and approved by the USDA and the Government of Madagascar. The United States Veterinary Permit for Importation and Transportation of Controlled Materials and Organisms and Vectors (Permit # 107234) was used. Although initially reviewed for approval by the Emory University’s Institutional Review Board, this work was subsequently determined to be ‘public health practice, with the goal of benefit to people in the region’ and therefore exempt from further human subjects review. In Madagascar, the IRB protocol was reviewed and approved by the Director of Health in the Fianarantsoa district. Both verbal and written consent were obtained for all participants 18 years and older. Verbal and written assent were obtained for those 10–17 years of age, and parents of children under the age of 10 acted as proxies for their children for the individual surveys.
Study site
This work was conducted in six rural villages bordering Ranomafana National Park (RNP) (21°02′–21°25′S, 47°18′–47°37′E) in the Ifanadiana District of Madagascar. RNP is a continuous humid tropical forest with natural vegetation ranging from montane cloud forest to lowland rainforest following an altitudinal gradient from 1513 to 600 metres above sea level [10].
Study procedures
Villages were defined as communities with at least 10 homes within 15 metres from one another, and are within ≤3 km of the boundaries of Ranomafana National Park. Six villages were randomly selected, and in each of the villages, 10 households were randomly selected to participate in the survey, malaria testing, and health assessment components of the study, for a total of sixty households. All age groups and sexes were eligible for testing. After completing informed consent forms, individuals were given health assessments measuring height, weight, temperature, blood pressure, and heart rate (Additional file 1). Individuals also received a malaria RDT (First Response Malaria Ag Combo Kits, Premier Med Corps, Nani Daman, India) to determine Plasmodium spp. infection. These tests are optimized to detect four malaria species: P. vivax, P. ovale, and P. malariae through a PAN test line specific to lactate dehydrogenase (pLDH), and P. falciparum through a Histidine-Rich Protein 2 (HRP2) specific to the species. Individuals testing positive for malaria were provided with recommended artemisinin combined therapy and advised to seek follow up care should symptoms persist. Individuals were also surveyed about insecticide-treated net (ITN) ownership and usage (Additional file 2).
Mosquitoes were trapped in the six villages and their associated agricultural and forest sites using two traps in each site. The two traps in the villages did not correspond to the households randomly selected for survey administration, but rather were placed on opposite ends of the village (Table 1) outside of households. Six traps were set per night for three consecutive nights in each village (four consecutive nights, battery permitting). Mosquitoes were trapped for a total of eighteen trapping nights at thirty-six trapping sites. Forest and agricultural sites were all <1 km from the village center. In most cases, land-use sites were contiguous (Table 1; Fig. 1). Agricultural sites were all former ‘tavy’ (slash-and-burn agriculture) sites where the forest has been burned and converted into rice paddies that are irrigated by rainfall. Forest sites included primary and secondary forest within national park boundaries. These sites had no human inhabitants and ranged from little (forest trails, trails to a few homes, etc.) to no daily human overlap (Fig. 1a).
Table 1.
Geographic coordinates and dates of sampling for mosquito trap sites in six villages near Ranomafana National Park, Madagascar
| Trap | Location | Elevation (m) | |
|---|---|---|---|
| Ambatolahy (6/17/13–6/21/13) | Forest | S 21°15′05.6″ E 047°25′21.6″ | 1011 |
| Forest w/odor | S 21°15′07.5″ E 047°25′23.3″ | 930 | |
| Village | S 21°14′57.3″ E 047°25′48.2″ | 865 | |
| Village w/odor | S 21°14′58.8″ E 047°25′47.3″ | 856 | |
| Agriculture | S 21°15′00.6″ E 047°25′48.1″ | 872 | |
| Agriculture w/odor | S 21°15′02.1″ E 047°25′47.7″ | 876 | |
| Vohiparara (6/30/13–7/4/13) | Forest | S 21°14′17.8″ E 047°23′41.4″ | 1129 |
| Forest w/odor | S 21°14′08.5″ E 047°23′46.9″ | 1129 | |
| Village | S 21°14′20.7″ E 047°22′53.0″ | 1133 | |
| Village w/odor | S 21°14′20.1″ E 047°22′54.7″ | 1136 | |
| Agriculture | S 21°14′11.4″ E 047°23′07.0″ | 1129 | |
| Agriculture w/odor | S 21°14′22.5″ E 047°22′58.2″ | 1131 | |
| Ambodiaviavy (7/7/13–7/10/13) | Forest | S 21°15′26.4″ E 047°28′34.1″ ± 10 m | 744 |
| Forest w/odor | S 21°15′23.4″ E 047°28′34.1″ ± 10 m | 781 | |
| Village | S 21°15′48.8″ E 047°29′06.0″ | 640 | |
| Village w/odor | S 21°15′50.8″ E 047°29′05.7″ | 642 | |
| Agriculture | S 21°15′45.6″ E 047°29′03.3″ | 623 | |
| Agriculture w/odor | S 21°15′50.4″ E 047°29′09.2″ | 619 | |
| Menarano (7/20/13–7/24/13) | Forest | S 21°17′27.9″ E 047°27′16.3″ | 834 |
| Forest w/odor | S 21°17′27.2″ E 047°27′20.2″ | 812 | |
| Village | S 21°17′26.3″ E 047°28′07.9″ | 716 | |
| Village w/odor | S 21°17′25.3″ E 047°28′07.2″ | 715 | |
| Agriculture | S 21°17′34.9″ E 047°28′06.5″ | 686 | |
| Agriculture w/odor | S 21°17′32.2″ E 047°27′59.6″ | 688 | |
| Manokoakora (7/29/13–8/1/13) | Forest | S 21°17′10.7″ E 047°32′46.1″ | 644 |
| Forest w/odor | S 21°17′11.0″ E 047°32′47.1″ | 646 | |
| Village | S 21°17′12.0″ E 047°32′36.1″ | 612 | |
| Village w/odor | S 21°17′12.5″ E 047°32′40.2″ | 612 | |
| Agriculture | S 21°17′17.7″ E 047°32′46.0″ | 605 | |
| Agriculture w/odor | S 21°17′15.0″ E 047°32′42.5″ | 616 | |
| Bevohazo (8/3/13–8/6/13) | Forest | S 21°12′20.4″ E 047°30′10.0″ | 720 |
| Forest w/odor | S 21°12′21.8″ E 047°30′07.0″ | 689 | |
| Village | S 21°12′37.0″ E 047°29′54.5″ | 616 | |
| Village w/odor | S 21°12′30.6″ E 047°29′54.7″ | 616 | |
| Agriculture | S 21°12′37.9″ E 047°29′52.0″ | 616 | |
| Agriculture w/odor | S 21°12′36.1″ E 047°29′54.4″ | 602 |
Fig. 1.

Examples of trapping sites in and around six villages near Ranomafana National Park, Madagascar. a Forest-trapping site. b Village-trapping site. c Agricultural-trapping site
Mosquitoes were collected from June through August 2013, using CDC miniature light traps (Model 512, John W. Hock Company, Gainesville, FL, USA) according to methods outlined in [14]. One of the two traps in each land-use site was randomly selected and baited with a synthetic human-derived odour, 3-Methyl-1-butanol (Fisher Scientific, Waltham, MA, USA catalog # 5001438080) [15] to improve capture of human malaria vectors, and field produced CO2 [14]. Each trap had a light sensor (LCS-2 Photo Switch, John W. Hock Company, Gainesville, FL, USA) attached that triggered the fan and light to turn on at sunset and off at sunrise, ensuring consistency among trapping sessions. Approximate distance of mosquito traps from the nearest livestock pen was also recorded.
Adult mosquitoes were identified morphologically on-site according to [16, 17] and stored in vials containing the desiccant Drierite (Fisher Scientific catalog # 075783B). All collected female Anopheles mosquitoes were dissected, and the head/thorax were separated to test for the presence of P.falciparum, P. vivax-210 and P. vivax-247 circumsporozoite proteins to determine malaria infection using the standard csELISA protocol as outlined in [18, 19]. These specific Plasmodium species were chosen because P. falciparum and P. vivax are the two most prevalent species of malaria in Madagascar [2].
Statistical analysis
Data analysis was performed using SAS 10.1 ® (SAS, Inc., Cary, North Carolina). General exploratory data analyses and bivariate relationships of variables of interest were examined. Poisson Regression models were used to examine the relationship between the prevalence of Anopheles and variables in the study that may influence mosquito prevalence. The independent variables included in the model were: land-use, village, odour, moon illumination, precipitation, temperature and elevation (Table 1). The dependent variable was Anopheles abundance. To examine whether land-use variability was altering overall mosquito captures or Anopheles specifically, the model was run to investigate the ratio of the number of Anopheles to the total number of mosquitoes by setting the total number of mosquitoes as the “offset” variable and Anopheles as the dependent variable in the model. Some data that were “missing at random” was left out of the analysis.
Results
The total number of female Anopheles mosquitoes captured in 36 sites (two traps in each land-use site, agriculture, village, and forest site, in six villages) was 414. The number of Anopheles mosquitoes captured in agricultural sites, village sites, and forest sites was 272, 124, and 18, respectively (Table 2). The most Anopheles mosquitoes and known vectors were captured in the village of Manoakoakora (n = 173) (Table 3).
Table 2.
Summary of Anopheles species captured, including known malaria vectors, in each land-use site in RNP, Madagacar
| Land-use site | Anopheles gambiae s.l. a | An. funestus a | An. mascarensis a | An. squamosus | An. coustani a | An. unknown | Total | Other mosquitoes |
|---|---|---|---|---|---|---|---|---|
| Agricultural | 169 | 61 | 15 | 0 | 2 | 25 | 272 | 610 |
| Village | 83 | 21 | 2 | 5 | 4 | 9 | 124 | 472 |
| Forest | 3 | 1 | 0 | 0 | 3 | 11 | 18 | 974 |
aKnown malaria vectors
Table 3.
Summary of Anopheles species captured, including known malaria vectors, in each of the six village-associated sites sampled in RNP, Madagacar
| Site | Anopheles gambiae s.l. a | An. funestus a | An. mascarensis a | An. squamosus | An. coustani a | An. unknown | Total | Other mosquitoes |
|---|---|---|---|---|---|---|---|---|
| Ambatolahy | 9 | 0 | 1 | 3 | 6 | 0 | 19 | 85 |
| Ambodiaviavy | 45 | 25 | 1 | 2 | 0 | 0 | 73 | 164 |
| Bevohazo | 27 | 9 | 2 | 0 | 0 | 2 | 40 | 730 |
| Manokoakora | 106 | 24 | 9 | 0 | 0 | 34 | 173 | 554 |
| Menarano | 68 | 22 | 3 | 0 | 3 | 6 | 102 | 190 |
| Vohiparara | 0 | 3 | 1 | 0 | 0 | 3 | 7 | 334 |
aKnown malaria vectors
Five Anopheles species were determined morphologically on-site, four of which are recognized malaria vectors: An. funestus s.l., An. gambiae s.l., An. mascarensis, and a recently recognized vector, An. coustani [3]. Anopheles squamosus, a non-vector was also identified, and the remaining Anopheles specimens were identified to the genus level, but not species (Tables 2, 3). Most of the Anopheles mosquitoes captured at agricultural sites were An. gambiae s.l. (62.1 %, n = 169), followed by An. funestus (22.4 %, n = 61).
The average percent of Anopheles of the total number of mosquitoes trapped each night in each village were ranged from 2.8 % (SE = 1.26) in Vohiparara, to 53.7 % (SE = 12.45) in Menarano (Table 4). The average percent of Anopheles of the total number of mosquitoes trapped each night at each land-use site was 1.6 % (SE = 0.91) in forest sites, 31.4 % (SE = 6.74) in village sites, and 41.4 % (SE = 7.96) in agricultural sites (Figs. 2, 3).
Table 4.
Summary and comparison of Anopheles and total mosquitoes trapped in each village per night in six villages near Ranomafana National Park, Madagascar
| Village | # Trap nights | Average # Anopheles trapped per night (SE) | Average # mosquitoes trapped per night (SE) | Average % Anopheles among total mosquitoes trapped per night (SE) |
|---|---|---|---|---|
| Ambatolahy | 4 | 7.8 (0.73) | 27.3 (1.42) | 28.4 (5.90) |
| Vohiparara | 4 | 1.8 (0.23) | 63.5 (5.02) | 2.8 (1.26) |
| Ambodiaviavy | 3 | 24.3 (6.21) | 63.0 (9.04) | 38.6 (13.20) |
| Menarano | 4 | 25.5 (4.35) | 47.5 (5.96) | 53.7 (12.45) |
| Manokoakora | 3 | 57.7 (5.39) | 193.3 (12.63) | 29.8 (9.00) |
| Bevohazo | 3 | 13.3 (2.20) | 253.3 (23.66) | 5.3 (4.84) |
Fig. 2.
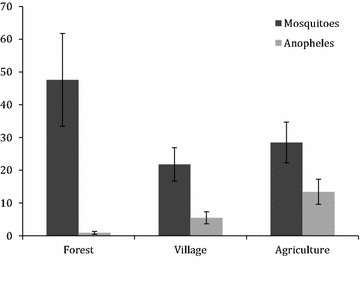
Average number of mosquitoes and Anopheles trapped each night by land-use type, with standard error bars, in six villages near Ranomafana National Park, Madagascar
Fig. 3.
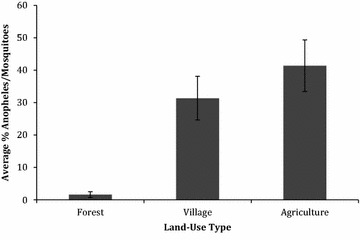
Average percent Anopheles of total mosquitoes trapped each night by land-use type, with standard error bars, in six villages near Ranomafana National Park, Madagascar
A regression model was run with the number of captured Anopheles mosquitoes as the outcome, and a type 3 analysis indicates that land-use is a significant predictor (p < 0.0001). A full Poisson regression model was run with all seven independent variables (land-use, odour, village, moon illumination, temperature, precipitation and proximity to animal pens), and the following were significant: land-use (p < 0.0001), village (p < 0.0001), odour (p < 0.0001), and proximity to livestock pens (p < 0.0001). When examining each land-use site separately, all categories (forest, village, agriculture) were significantly different from one another. The expected log counts of Anopheles for village and forest sites were 0.63- and 1.95-fold lower than those from agricultural sites. When examining the relationship between the ratio of Anopheles to total number of mosquitoes captured and the independent variables listed above, the following variables were significant: land-use (p < 0.0001), village (p < 0.0001), proximity to animals (p < 0.0001) and moon illumination (p = 0.0047). Traps located in close proximity (<3 m) to livestock pens were 2.62 times more likely (p < 0.0001) to capture Anopheles mosquitoes than those traps that were not in close proximity to animal pens.
All 414 female Anopheles mosquitoes were individually screened for the circumsporozoite protein using the well-established csELISA protocol [17, 18]. Three mosquitoes (0.7 % of total captured), were found positive for P. vivax-210 circumsporozoite proteins. These mosquitoes were found in An. gambiae s.l. and An. funestus and trapped in three different locations: An. funestus in Manokoakora agricultural site, An. gambiae s.l. in Manokoakora village site, and An. gambie s.l. in Bevohazo village site.
Out of a total of 305 study participants, 272 individuals consented to malaria RDTs. 3.7 % were positive for P. falciparum (Table 5). No individuals tested positive for PAN Plasmodium (P. vivax, P. ovale, P. malariae). All positive individuals were from different households, and P. falciparum infection was detected in five of the six villages surveyed (Table 6). None of the positive individuals were febrile during the health assessments; however, several did report low-grade symptoms (i.e., body aches). Seven of the ten positive individuals were females aged: 2, 2.5, 8, 16, 23, 26, and 35 years. One female was post-partum. The three males that tested positive were 7, 9, and 21 years of age.
Table 5.
Summary of human population demographics, nurse assessments, and ITN ownership and usage as recorded in this study
| Characteristic | N (%) | Total Na |
|---|---|---|
| Demographics | ||
| Sex | 305 | |
| Female | 174 (57.0) | |
| Male | 131 (43.0) | |
| Age (years) | 303 | |
| <5 | 50 (16.5) | |
| 5 to 17 | 113 (37.3) | |
| ≥18 | 142 (46.9) | |
| Nurse assesments | ||
| BMIb (<18.5) | 13 (11.8) | 110 |
| Stuntingc (< −2 SD) | 10 (20.0) | 50 |
| Underweight2 (< −3 SD) | 17 (34.0) | 50 |
| Febrile (temp > 100.4° F) | 3 (1.1) | 265 |
| Positive malaria RDT | 10 (3.7) | 272 |
| Insecticide Treated Net (ITN) ownership | ||
| Live in house with ITN | 315 (94.3) | 334 |
| If yes, number of ITNs | ||
| 1 | 36 (11.4) | 315 |
| 2 | 115 (36.5) | 315 |
| 3 | 125 (39.7) | 315 |
| 4 | 31 (9.8) | 315 |
| 5 | 0 (0.0) | 315 |
| 6 | 8 (2.5) | 315 |
| ITN Usage | ||
| Never | 70 (23.3) | 301 |
| Children <5 | 10 (3.3) | |
| Females ≥18 | 18 (6.0) | |
| Not every night | 15 (5.0) | 301 |
| Children <5 | 2 (0.07) | |
| Females ≥18 | 4 (1.3) | |
| Every night | 216 (71.8) | 301 |
| Children <5 | 37 (1.2) | |
| Females ≥18 | 59 (2.0) | |
aNot all individuals were willing to participate in all components of survey and health assessments, therefore total n is listed
bBMI reported for the ≥18 year-old population who completed the physical assessment
cStunting and underweight reported for <5 year-old population
Table 6.
Summary of human cases of P. falciparum found in six villages sampled near Ranomafana National Park, Madagascar
| Village | Positive P. falciparum (%) | Household members (n) | Malaria RDTs administered (%) |
|---|---|---|---|
| Ambatolahy | 2.2 (n = 1) | 56 | 80.3 (n = 45) |
| Ambodiaviavy | 2.1 (n = 1) | 53 | 71.7 (n = 38) |
| Bevohazo | 5.1 (n = 3) | 58 | 94.8 (n = 55) |
| Manokoakora | 2.1 (n = 1) | 52 | 88.5 (n = 46) |
| Menarano | 8.5 (n = 4) | 68 | 69.1 (n = 47) |
| Vohiparara | 0.0 (n = 0) | 47 | 87.2 (n = 41) |
When questioned about ITN ownership, 94.3 % of individuals lived in households with ITNs (Table 5). When asked how often individuals slept under a bed net over the past four weeks (Additional file 2), 70 (23.3 %) reported never sleeping under a bed net. Of the individuals that never sleep under ITNs, 10 (14.3 %) were children under the age of 5, and 18 (25.7 %) were women over the age of 18 (Table 5).
Discussion
In forested areas of Madagascar, land-use plays a critical role in Anopheles abundance, with highest Anopheles abundance in agricultural sites followed by village and forested sites. More malaria vectors, An. gambiae s.l. (62.1 %, n = 169), were trapped in agricultural sites in this study than in any other land-use site. Certain agricultural practices, such as the irrigation of rice fields in our agricultural study sites, may increase the number of breeding of mosquitoes [9], which can lead to an increase in malaria prevalence [3]. It is possible that when converting land from forests into rice cultivation sites Madagascar, the irrigation structure using rainfall to flood rice fields creates pools of stagnant water with small amounts of surface vegetation, creating ideal breeding habitats for Anopheles [20, 21].
Mosquito traps set within <3 m of livestock pens were significantly more likely (p < 0.0001) to capture Anopheles mosquitoes than those far from livestock pens. In a previous study in Madagascar, An. gambiae and An. arabiensis showed an innate preference for calf odour over human odour [22], perhaps providing an explanation for our capture numbers near livestock pens. Another study suggested that An. arabiensis exhibits a high degree of zoophily in Madagascar [23], which may also explain these livestock associated capture numbers. In this study, however, An. gambiae s.l. and An. arabiensis were not differentiated, so it cannot be assumed that Anopheles caught near livestock pens were indeed An. arabiensis. Even if zoophilic, An. arabiensis, a well-recognized malaria vector, is a threat to human health, and may pose risk to humans tending to livestock.
The large number of cattle in Madagascar may also increase the number of dead end hosts for human malaria, and therefore actually decrease overall malaria transmission in the area. This concept is known as zooprophylaxis [24]. Further research investigating the role of cattle and human Plasmodium spp. infection in this region will elucidate whether or not cattle ownership has potential use as a form of malaria vector control, or if it increases human exposure to malaria vectors. Whether protecting human inhabitants through zooprophylaxis, or attracting malaria mosquitoes to human inhabitants as found in [25], livestock placement and husbandry practices should be incorporated into malaria prevention and intervention strategies. Targeted insecticide control for humans as well as livestock may provide additional protection against malaria carrying mosquitoes in Madagascar.
In this study, known malaria vectors (An. gambiae s.l., An. funestus, An. mascariensis, and An. coustani) were found to make up 90.1 % of Anopheles spp. captured in agricultural land-use sites during the months of June–August, suggesting that these sites may be high-risk areas for malaria. Such geographic data on parasite vectors can inform and greatly improve malaria control and elimination programmes [26].
When screening captured mosquitoes for malaria parasites, 0.7 % were positive for malaria species P. vivax-210. One of the villages sampled, Manokoakora, differed from the others in that gold extraction has become common practice in this village and its surrounding agricultural fields. The method of extraction involves digging deep holes that often remain full of still standing water, and vegetation from rice cultivation, which may create an optimal Anopheles habitat. This land-use modification may explain why 41.8 % (n = 173) of all Anopheles in this study were caught in Manokoakora (Table 3), and two of the three mosquitoes that tested positive for P. vivax-210, were captured in and around this village. While this is a low number, and may be due to a larger Anopheles sample size in this village, with the expansion of gold-mining practices in RNP, further research examining the significance of gold mining on mosquito-borne diseases in the region is necessary.
Unlike the P. vivax-210 detected in mosquitoes, when using RDTs, only P. falciparum was detected in associated human populations. Historically, it was thought that erythrocyte Duffy blood group negative individuals, mainly of African ancestry, are resistant to P. vivax infection [27], due to the Duffy protein acting as an entrance point into erythrocytes. Previous studies have identified Duffy negative individuals in Madagascar infected with P. vivax, suggesting that in some regions of Madagascar, P. vivax is no longer dependent on the Duffy antigen for establishing human infection and disease [27]. Future work in the RNP region has the potential to reveal whether or not the populations in this region are Duffy negative and hence “resistant” to P. vivax infection, perhaps explaining why P. vivax infection was identified in mosquito vectors in this study, but not in the human populations. Other potential explanations for this discrepancy between human and mosquito infections could be due to false positives in mosquitoes [28], missed P. vivax human infections due to low sensitivity (possibly due to low detection level limits) of the RDT used, or that the number of people tested was so low that P. vivax could not be detected.
Most individuals surveyed in this study reported ownership and usage of ITNs (Table 5), which likely plays an important role in the protection of humans in the region from malaria; however, not all individuals in target demographics (children under the age of five and women over the age of 18) reported sleeping under ITNs regularly (Table 5). Since malaria has the highest fatality rates in women and children under the age of five, it is crucial to emphasize the importance of regular ITN usage in the region, especially for these individuals.
Conclusions
By combining human health assessments and surveys with environmental vector sampling in varying land-use sites in rural Madagascar, this study revealed that malaria vectors are abundant in agricultural land-use sites, in particular in proximity to animal pens, and that humans in the region test positive for P. falciparum in the winter months (Jun–Aug), while P. vivax 210 is detected in mosquitoes, and 23.3 % of individuals report never using ITNs. A deeper understanding of the locations where malaria vectors are prevalent, such as the agricultural land-use sites or near livestock pens presented in this study, is necessary to improve targeted malaria prevention strategies in the region.
Authors’ contributions
SZ, KSD, EGH, PCW, and TRG conceived of the project. SZ, KSD, MTA, EGH, and TRG conducted the fieldwork and laboratory analyses. SZ, KSD, and TRG conducted statistical analyses, and all authors contributed to writing and editing the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful for logistical and infrastructural support from MICET, particularly director Benjamin Andriamihaja, the administration and support personnel of the Centre ValBio, Madagascar National Parks, Eileen Larney, John Cadle, Rabaovola Bernadette, Heritiana Anne Louisette, Velonabison Mamitiana Jean José, Razafindraibe Faustin Jean Guy, Rakotonjatovo Justin, and Razafindraibe Faustin Jean Guy, Cassidy Rist, Morgan Mercer, Paul Kennedy, Alice Sutcliffe, and Caroline Schwaner. We thank two anonymous reviewers for their feedback and suggestions in improving this manuscript. This research was supported by the Jim and Robin Herrnstein Foundation, the Emory University Global Health Institute, the Center for Disease Control and Prevention Malaria Research and Reference Reagent and Resource Center (MR4), and NIH/NIGMS IRACD Grant K12 GM000680.
Competing interests
The authors declare that they have no competing interests.
Additional files
10.1186/s12936-016-1164-2 Individual health assessment form.
10.1186/s12936-016-1164-2 Examples of survey questions asked regarding ITN ownership and usage.
Contributor Information
Sarah Zohdy, Email: sarahzohdy@gmail.com.
Kristin Derfus, Email: kristin.derfus@gmail.com.
Emily G. Headrick, Email: emilygayle@gmail.com
Mbolatiana Tovo Andrianjafy, Email: amthta@gmail.com.
Patricia C. Wright, Email: patchapplewright@gmail.com
Thomas R. Gillespie, Email: thomas.gillespie@emory.edu
References
- 1.PMI. Malaria operational plan fy 2014: Madagascar. http://www.pmi.gov/countries/mops/fy14/madagascar_mop_fy14.pdf. Accessed 8 Feb 2016.
- 2.WHO. World Malaria Report 2012: Madagascar. Geneva: World Health Organization; 2012. http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_no_profiles.pdf. Accessed 8 Feb 2016.
- 3.Nepomichene TN, Tata E, Boyer S. Malaria case in Madagascar, probable implication of a new vector, Anopheles coustani. Malar J. 2015;14:1. doi: 10.1186/s12936-015-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnadas C, Tichit M, Bouchier C, Ratsimbasoa A, Randrianasolo L, Raherinjafy R, et al. Plasmodium vivax dhfr and dhps mutations in isolates from Madagascar and therapeutic response to sulphadoxine-pyrimethamine. Malar J. 2008;7:35. doi: 10.1186/1475-2875-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutero CM, Amerasinghe F, Boelee E, Konradsen F, Van der Hoek W, Nevondo T, et al. Systemwide initiative on malaria and agriculture: an innovative framework for research and capacity building. Eco Health J. 2005;2:11–16. [Google Scholar]
- 6.Utzinger J, Tozan Y, Singer BH. Efficacy and cost-effectiveness of environmental management for malaria control. Trop Med Int Health. 2001;6:677–687. doi: 10.1046/j.1365-3156.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 7.Sussman RW, Green GM, Sussman LK. Satellite imagery, human ecology, anthropology, and deforestation in Madagascar. J Hum Ecol. 1994;22:333–354. doi: 10.1007/BF02168856. [DOI] [Google Scholar]
- 8.Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/S0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 9.Pongsiri MJ, Roman J, Ezenwa VO, Goldberg TL, Koren HS, Newbold SC, et al. Biodiversity loss affects global disease ecology. Bioscience. 2009;59:945–954. doi: 10.1525/bio.2009.59.11.6. [DOI] [Google Scholar]
- 10.Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- 11.Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Wagner S. Linking deforestation to malaria in the amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12. [PMC free article] [PubMed] [Google Scholar]
- 12.Wright PC. The Future of Biodiversity in Madagascar: A View from Ranomafana National Park. In: Patterson BD, Goodman SM, editors. Natural Change and Human Impact in Madagascar. Washington DC: Smithsonian University Press; 1997. pp. 381–405. [Google Scholar]
- 13.Brooks CP, Holmes C, Kramer K, Barnett B, Keitt TH. The role of demography and markets in determining deforestation rates near Ranomafana National Park. Madagascar PLoS One. 2009;4:e5783. doi: 10.1371/journal.pone.0005783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zohdy S, Derfus K, Andrianjafy MT, Wright PC, Gillespie TR. Field evaluation of synthetic lure (3-methyl-1-butanol) when compared to non odour-baited control in capturing Anopheles mosquitoes in varying land-use sites in Madagascar. Parasit Vectors. 2015;8:145. doi: 10.1186/s13071-015-0729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, van Loon JJ, et al. A novel synthetic odourant blend for trapping of malaria and other African mosquito species. J Chem Ecol. 2012;38:235–244. doi: 10.1007/s10886-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillies MT, de Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) Publ S Afr Inst Med Res. 1968;54:1–343. [Google Scholar]
- 17.Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–143. [Google Scholar]
- 18.Wirtz R, Avery M, Benedict M. Chapter 3: Specific anopheles techniques 3.3 Plasmodium falciparum sporozoite ELISA. Methods in Anopheles research (2nd edn). National Institutes of Health, NIAID MR4. 2011.
- 19.Burkot TR, Williams JL, Schneider I. Identification of Plasmodium falciparum-infected mosquitoes by a double antibody enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1984;33:783–788. doi: 10.4269/ajtmh.1984.33.783. [DOI] [PubMed] [Google Scholar]
- 20.Junglen S, Kurth A, Kuehl H, Quan PL, Ellerbrok H, Pauli G, et al. Examining landscape factors influencing relative distribution of mosquito genera and frequency of virus infection. EcoHealth. 2009;6:239–249. doi: 10.1007/s10393-009-0260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillinger U, Sonye G, Killeen GF, Knols BG, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop Med Int Health. 2004;9:1274–1289. doi: 10.1111/j.1365-3156.2004.01335.x. [DOI] [PubMed] [Google Scholar]
- 22.Duchemin JB, Tsy JM, Rabarison P, Roux J, Coluzzi M, Costantini C. Zoophily of Anopheles arabiensis and An. gambiae in Madagascar demonstrated by odour-baited entry traps. Med Vet Entomol. 2001;15:50–57. doi: 10.1046/j.1365-2915.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 23.Fontenille D, Lepers JP, Campbell GH, Coluzzi M, Rakotoarivony I, Coulanges P. Malaria Malaria transmission and vector biology in Manarintsoa, high plateaux of Madagascar. Am J Trop Med Hyg. 1990;43:107–115. doi: 10.4269/ajtmh.1990.43.107. [DOI] [PubMed] [Google Scholar]
- 24.Saul A. Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar J. 2003;2:32. doi: 10.1186/1475-2875-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouma M, Rowland M. Failure of passive zooprophylaxis: cattle ownership in Pakistan is associated with a higher prevalence of malaria. Trans R Soc Trop Med Hyg. 1995;89(4):351–353. doi: 10.1016/0035-9203(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 26.Mendis K, Rietveld A, Warsam M, Bosman A, Greenwood B, Wernsdorfer WH. From malaria control to eradication: the WHO perspective. Trop Med Int Health. 2009;14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- 27.Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci USA. 2010;107:5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195. doi: 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]


