Abstract
Background: Reconstruction of soft tissue defects has traditionally relied on the use of grafts and flaps, which may be associated with variable resorption and/or significant donor site morbidity. Cell-based strategies employing adipose-derived stromal cells (ASCs), found within the stromal vascular fraction (SVF) of adipose tissue, may offer an alternative strategy for soft tissue reconstruction. In this study, we investigated the potential of a bone morphogenetic protein receptor type 1A (BMPR1A)(+) subpopulation of ASCs to enhance de novo adipogenesis.
Methods: Human lipoaspirate was enzymatically digested to isolate SVF and magnetic-activated cell separation was utilized to obtain BMPR1A(+) and BMPR1A(−) cells. These cells, along with unenriched cells, were expanded in culture and evaluated for adipogenic gene expression and in vitro adipocyte formation. Cells from each group were also labeled with a green fluorescent protein (GFP) lentivirus and transplanted into the inguinal fat pads, an adipogenic niche, of immunocompromised mice to determine their potential for de novo adipogenesis. Confocal microscopy along with staining of lipid droplets and vasculature was performed to evaluate the formation of mature adipocytes by transplanted cells.
Results: In comparison to BMPR1A(−) and unenriched ASCs, BMPR1A(+) cells demonstrated significantly enhanced adipogenesis when cultured in an adipogenic differentiation medium, as evidenced by increased staining with Oil Red O and increased expression of peroxisome proliferator-activating receptor gamma (PPAR-γ) and fatty acid-binding protein 4 (FABP4). BMPR1A(+) cells also formed significantly more adipocytes in vivo, as demonstrated by quantification of GFP+ adipocytes. Minimal formation of mature adipocytes was appreciated by BMPR1A(−) cells.
Conclusions: BMPR1A(+) ASCs show an enhanced ability for adipogenesis in vitro, as shown by gene expression and histological staining. Furthermore, within an adipogenic niche, BMPR1A(+) cells possessed an increased capacity to generate de novo fat compared to BMPR1A(−) and unenriched cells. This suggests utility for the BMPR1A(+) subpopulation in cell-based strategies for soft tissue reconstruction.
Introduction
The stromal vascular fraction (SVF) obtained from human lipoaspirate contains numerous cell populations, most notably adipose-derived stromal cells (ASCs).1 Their broad differentiation capability makes ASCs attractive candidates for use in the development of new surgical therapies, with their adipogenic potential particularly useful for soft tissue reconstruction. While ASCs have shown potential for improving the survival of both ischemic flaps and fat grafts, their ability for de novo adipogenesis represents an alternative means for restoration of contour deformities.2–4 This has been demonstrated by Tsuji et al., who observed that ASCs seeded on a type I collagen sponge formed fat in vivo when implanted into immunocompromised mice.5
As ASCs are a heterogeneous mesenchymal stem/progenitor cell population, enrichment for subpopulations with enhanced differentiation potential is a promising strategy for the development of targeted cell-based therapies. For example, ASCs with decreased expression of CD105 demonstrate enhanced osteogenic potential both in vitro and in vivo in comparison to both CD105+ and unenriched cells.6 Similarly, increased expression of cell surface markers CD90 and bone morphogenetic protein receptor type 1B (BMPR1B) have been found to also identify pro-osteogenic ASC subpopulations.7,8 Use of cells enriched for the presence of these markers may thus facilitate more efficient bone formation in the context of surgical therapies for the treatment of skeletal defects.
In contrast to pro-osteogenic ASC subpopulations, proadipogenic subpopulations have been relatively less characterized. Li et al. evaluated the adipogenic potential of the SVF cell subpopulations characterized by CD34, CD31, and CD146 expression (among CD45− cells), determining that the CD34+/CD31− subfraction of SVF possesses the highest adipogenic potential.9 Although they referred to this subpopulation as a preadipocyte population, the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) have stated that the pattern of CD45−/CD34+/CD31− surface marker expression defines ASCs within SVF.9,10 Thus, a true proadipogenic subpopulation of ASCs remains to be identified.
Members of the transforming growth factor beta superfamily, BMPs and their receptors have been implicated in the lineage commitment of mesenchymal stem cells, with BMPR1A specifically linked to adipogenesis: Chen et al. found that overexpression of a constitutively active form of BMPR1A in 2T3 cells was sufficient to induce adipocyte formation, while Huang et al. observed similar results in C3H10T1/2 cells.11,12 Both BMP2 and BMP4 are ligands for BMPR1A, and addition of BMP2 to the adipogenic differentiation medium (ADM) has been found to enhance in vitro adipogenic differentiation of ASCs.13 Given these findings, we reasoned that enrichment of ASCs based on BMPR1A expression might yield a putatively proadipogenic subpopulation of ASCs. Herein, we demonstrate that this is in fact the case, and that enrichment of cells for BMPR1A expression identifies a candidate population for use in the development of treatments aimed at de novo adipogenesis for soft tissue reconstruction.
Materials and Methods
Cell harvest and culture
Fresh human lipoaspirate was collected from four healthy female donors (ages 35–56) with no medical comorbidities after obtaining informed consent, in accordance with a Stanford Institutional Review Board approved protocol (no. 2188). Lipoaspirate was washed with phosphate-buffered saline (PBS) and digested with 0.075% type II collagenase (Sigma-Aldrich Co. LLC) as previously described.14 Cells were cultured in a standard growth medium consisting of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and Primocin™ (100 μg/mL; InvivoGen), at 37°C/5% CO2. To obtain necessary numbers of cells for all experiments, cells were passaged up to four times over the course of 1 month.
Magnetic-activated cell sorting and flow cytometry
BMPR1A(+)-enriched and BMPR1A(−)-enriched cell populations were isolated from freshly harvested SVF using magnetic-activated cell sorting (MACS). Briefly, cells were first washed in a staining buffer (PBS/2% FBS/1% penicillin–streptomycin), then incubated at 4°C for 20 min in a solution containing biotinylated anti-human BMPR1A antibody (1:20; R&D Biosystems). Cells were then washed and centrifuged before resuspension and further incubation with Anti-Biotin MicroBeads (Miltenyi Biotec, Inc.) at a 1:5 dilution in a staining buffer. To verify successful enrichment using fluorescence activated cell sorting (FACS) analysis, cells were subsequently incubated with Anti-Biotin-PerCP-Vio700 (Miltenyi Biotec, Inc.) at a 1:10 dilution in a staining buffer for 5 min. Labeled cells were separated using MACS LS Columns (Miltenyi Biotec, Inc.) as per manufacturer's instructions. FACS analysis was performed using a BD FACSAria II (BD Biosciences), with the proportion of BMPR1A(+) cells evaluated after gating to exclude debris based on forward and side scatter.
XTT assay
Passage-one BMPR1A(+), BMPR1A(−), and unenriched ASCs were seeded at a density of 4000 cells per well in a 96-well tissue culture plate, with replicates of 7 wells per group. An 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt (XTT) assay was performed as per the manufacturer's instructions at three time points over a 24-h period (Cell Proliferation Kit II [XTT]; Roche Applied Science).
In vitro adipogenic differentiation
The in vitro adipogenic capacity of BMPR1A(+), BMPR1A(−), and unenriched cells was assessed by culturing cells in 12-well culture dishes in ADM consisting of DMEM, 10% FBS, 100 μg/mL Primocin, 10 μg/mL insulin, 1 μM dexamethasone, 0.5 mM methylxanthine, 200 μM indomethacin, and with (ADM+BMP2) or without (ADM) the addition of 100 ng/mL recombinant human BMP2 (Thermo Fisher Scientific, Inc.), as described by Lee et al.13 Cells were seeded at a density of 50,000 cells per well of a 12-well plate, with adipogenic assays performed on subconfluent cells in triplicate wells to facilitate statistical analysis. Oil Red O (ORO) staining was performed after 7 days of differentiation. After imaging, isopropanol was used to extract the stain from each well, and the absorbance at 495 nm measured.
Reverse transcription and quantitative real-time polymerase chain reaction
RNA was extracted from cells that underwent treatment with ADM or ADM+BMP2, for 7 days, as well as those cultured in the standard growth medium, using an RNeasy Mini Kit (Qiagen). Reverse transcription was performed and adipogenic gene expression analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) using the Applied Biosystems Prism 7900HT sequence detection system (Applied Biosystems). The quantity of each gene expressed was normalized to the quantity of β-actin expression in each sample. Specific primer sequences used are shown in Table 1.
Table 1.
Primers Used for Quantitative Real-Time Polymerase Chain Reaction
| Gene | Forward primer sequence | Reverse primer sequence | PrimerBank ID |
|---|---|---|---|
| PPAR-γ | TACTGTCGGTTTCAGAAATGCC | GTCAGCGGACTCTGGATTCAG | 116284372c3 |
| FABP4 | ACTGGGCCAGGAATTTGACG | CTCGTGGAAGTGACGCCTT | 168480125c1 |
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT | 4501885a1 |
All primer sequences were obtained from PrimerBank (The Massachusetts General Hospital, Boston, MA).
FABP4, fatty acid-binding protein 4; PPAR-γ, peroxisome proliferator-activating receptor gamma.
Cell labeling and in vivo adipogenic differentiation
To label cells for in vivo experiments, BMPR1A(+), BMPR1A(−), and unenriched ASCs were transduced with a green fluorescent protein (GFP)-encoding lentivirus (Santa Cruz Biotechnology, Inc.) and underwent puromycin selection to obtain pure populations of transduced cells. Transduced BMPR1A(+), BMPR1A(−), and unenriched cells were injected into the inguinal fat pads of immunocompromised mice. All experiments were performed in accordance with the Stanford University Administrative Panel on Laboratory Animal Care.
Briefly, GFP-expressing cells from each group were lifted, counted, and divided into aliquots of 500,000 cells. Pelleted cells were resuspended in a 10% type B gelatin solution (Sigma-Aldrich). The inguinal fat pads of 10-week-old Crl:CD-1-Foxn1nu mice (Charles River Laboratories) were injected with suspensions containing either BMPR1A(+), BMPR1A(−), or unenriched ASCs, with four mice injected per group. A total of 50,000 cells were injected per inguinal fat pad for each group of mice. Additionally, a separate group of four mice received injections with 10% type B gelatin alone. Mice were sacrificed 4 weeks after injection, and their inguinal fat pads explanted for histological analysis.
Histological analysis
Explanted inguinal fat pads were fixed in 4% paraformaldehyde overnight and subsequently washed in PBS. Fat pads were minced and stained with Isolectin GS-IB4 Alexa Fluor® 594 Conjugate (1:100; Thermo Fisher Scientific, Inc.) to evaluate vasculature, HCS LipidTOX™ Deep Red neutral lipid stain (1:100; Thermo Fisher Scientific, Inc.) for staining of lipid droplets, and DAPI (1:10,000; Thermo Fisher Scientific, Inc.) for labeling of cell nuclei. Stained fat pads were then processed as described by Berry et al.15 Whole-mount imaging was performed using a Leica WLL SP8 confocal microscope. Cells derived from implanted cells were identified by positive GFP signal. The proportion of GFP+ adipocytes was manually quantified for each group (n = 3 images at 20× magnification per group).
Statistical analyses
Statistical analyses were performed using a one-way analysis of variance (ANOVA) for comparisons of multiple groups, with Tukey's test used for post hoc analysis. Two-tailed Student's t-tests were used for direct comparisons between two groups. A two-way ANOVA with Tukey's multiple comparisons test was used for analysis of XTT assay data. A p-value <0.05 was considered significant. All data are presented as mean ± standard deviation.
Results
BMPR1A(+) ASCs demonstrate superior adipogenesis in vitro
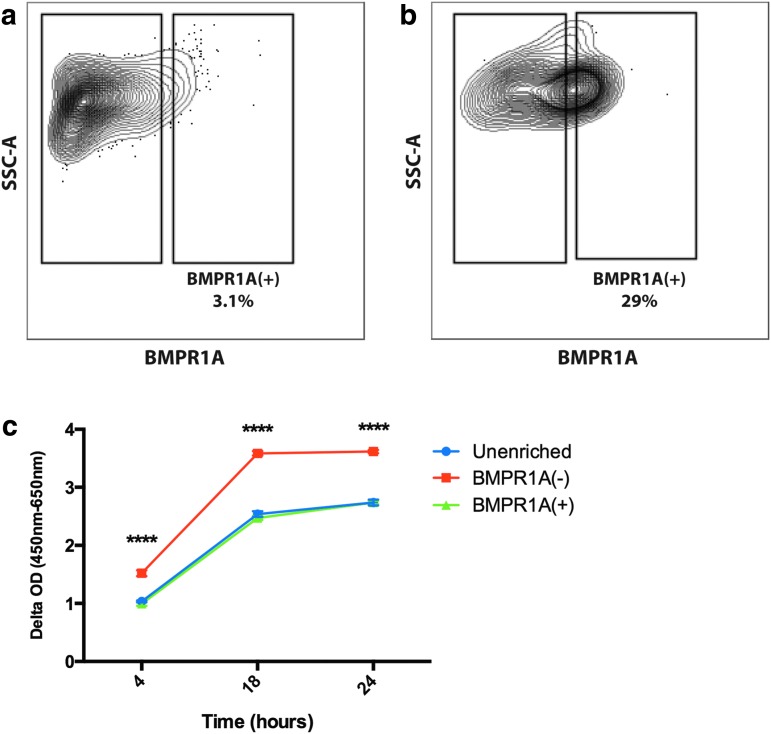
FACS analysis of unsorted cells demonstrated 3.1% to be BMPR1A(+) (Fig. 1a). This increased to 29% through MACS enrichment (Fig. 1b). XTT assay showed that over 24 h in culture, BMPR1A(−) cells were significantly more proliferative compared to BMPR1A(+) and unenriched ASCs (****p ≤ 0.0001) (Fig. 1c).
FIG. 1.
FACS analysis of freshly harvested SVF cells. (a) Unenriched SVF contains ∼3.1% BMPR1A(+) cells, while (b) analysis of magnetic activated cell sorting-enriched cells shows that ∼29% are BMPR1A(+). (c) XTT assay showed similar proliferation ability of BMPR1A(+) and unenriched ASCs, while BMPR1A(−) cells showed significantly greater proliferation in vitro (****p < 0.0001). ASCs, adipose-derived stromal cells; BMPR1A, bone morphogenetic protein receptor type 1A; SVF, stromal vascular fraction; FACS, fluorescence activated cell sorting.
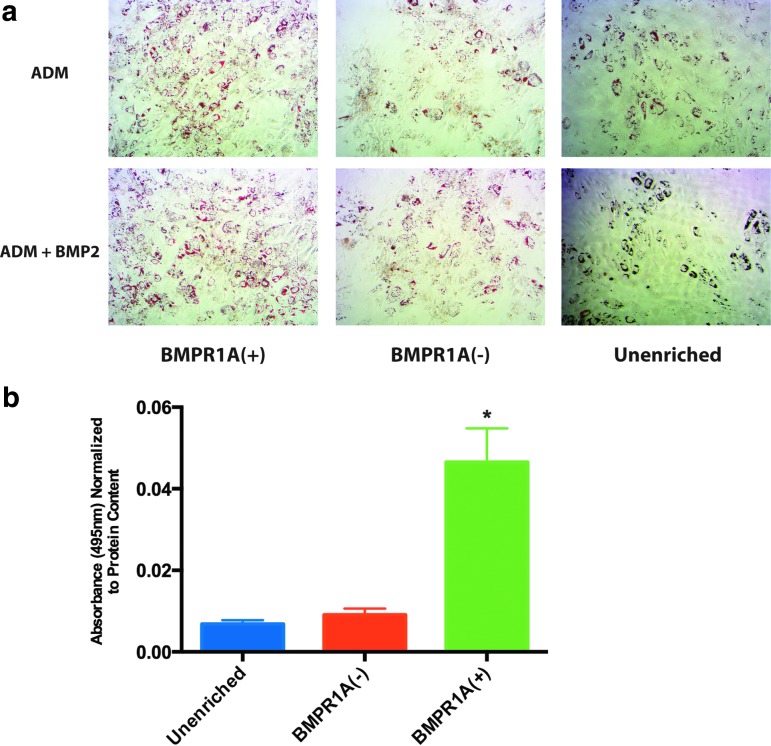
In vitro adipogenic potential of BMPR1A(+)-enriched cells, BMPR1A(−) cells, and unenriched cells was determined by culture for 7 days in ADM and ADM+BMP2, after which cells were either stained with ORO for histological analysis of adipocyte formation, or RNA was extracted for assessment of adipogenic gene expression. BMPR1A(+) cells demonstrated increased in vitro adipocyte formation in comparison to both of the other cell populations, as evidenced by increased ORO staining relative to both BMPR1A(−) and unenriched cells after culture in both ADM and ADM+BMP2 (Fig. 2a), although these differences were statistically significant only for cells cultured in ADM+BMP2 (*p < 0.05) (quantification of ADM ORO staining not shown) (Fig. 2b).
FIG. 2.
Adipogenic differentiation of ASCs enriched for BMPR1A in comparison to BMPR1A(−) and unenriched cells. (a) ORO staining of ASCs after 7 days of culture in ADM and ADM+BMP2. All images are at 10× magnification. (b) Quantification of ORO staining based on absorbance of extracted stain at 495 nm. BMPR1A(+) ASCs demonstrate significantly increased staining (*p < 0.05) compared to both unenriched and BMPR1A(−) cells after culture in ADM+BMP2. ADM, adipogenic differentiation medium; ORO, Oil Red O.
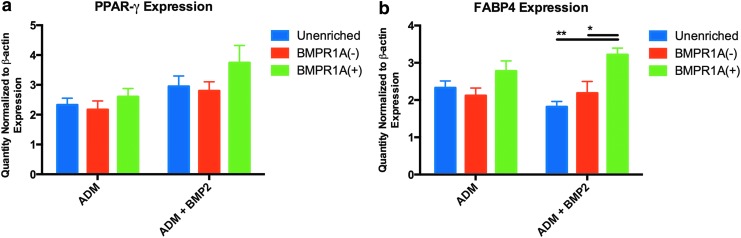
We expanded on these observations by using qRT-PCR analysis to evaluate adipogenic gene expression. BMPR1A(+)-enriched cells demonstrated greater upregulation of adipogenic markers than both BMPR1A(−) and unenriched cells in response to treatment with both ADM and ADM+BMP2: absolute levels of peroxisome proliferator-activating receptor gamma (PPAR-γ) were higher in BMPR1A(+) cells than in both BMPR1A(−) and unenriched ASCs (Fig. 3a). As expected, expression of fatty acid-binding protein 4 (FABP4), a target gene of PPAR-γ expressed by terminally differentiated cells, was highest in BMPR1A(+) ASCs, and significantly higher than both BMPR1A(−) (*p < 0.05) and unenriched cells after culture in ADM+BMP2 (**p < 0.01) (Fig. 3b).16,17
FIG. 3.
After 7 days of culture in ADM and ADM+BMP2, (a) BMPR1A(+) cells demonstrated greater expression of PPAR-γ than both BMPR1A(−) and unenriched ASCs, while (b) levels of FABP4 expression were significantly increased in BMPR1A(+) cells in comparison to unenriched (**p < 0.01) and BMPR1A(−) (*p < 0.05) populations after culture in ADM+BMP2. FABP4, fatty acid-binding protein 4; PPAR-γ, peroxisome proliferator-activating receptor gamma.
BMPR1A(+) ASCs demonstrate enhanced adipogenesis in vivo
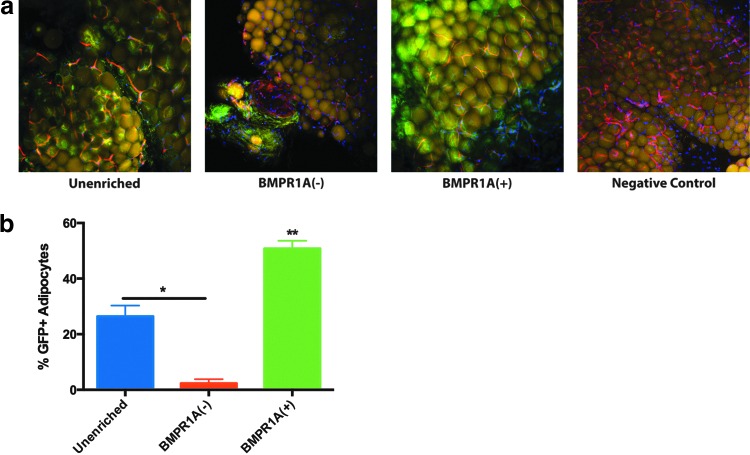
While BMPR1A(+) cells demonstrated a superior capacity for adipogenic differentiation in vitro, it was necessary to evaluate whether or not this potential was translatable in vivo. To do this, we injected GFP-expressing cells into the inguinal fat pads of immunocompromised mice (a nonhypoxic, naturally proadipogenic microenvironment). Four weeks after transplantation, GFP+ cells could be visualized in whole-mount sections by confocal microscopy (Fig. 4a). Paralleling our in vitro data, BMPR1A(+) ASCs showed superior adipogenic ability in vivo, with a significantly larger percent of GFP+ adipocytes observed in inguinal fat pads that had received these cells (**p < 0.05), as identified by GFP overlap with far red (HCS LipidTOX) signal (Fig. 4b). While unenriched ASCs also successfully formed adipocytes in the inguinal fat pad, significantly fewer GFP+ adipocytes were appreciable in sections from fat pads transplanted with BMPR1A(−) ASCs (*p < 0.05) (Fig. 4b). Instead, BMPR1A(−) cells appeared to form fibrous tissue and localize to the perivascular regions of the adipose tissue (Fig. 4a).
FIG. 4.
In vivo adipogenesis as assessed by transplanting GFP-expressing cells into the inguinal fat pads of immunocompromised mice. (a) Images obtained by confocal microscopy at 20×. Lipid droplets (yellow) are stained with HCS LipidTOX, vasculature (red) is stained with Isolectin, and cell nuclei (blue) are stained with DAPI. Negative Control refers to inguinal fat pads receiving injections of 10% type B gelatin solution alone. (b) Quantification of the percent of total adipocytes that are GFP+ facilitated assessment of the amount of adipogenic differentiation by transplanted cells, and BMPR1A(+) ASCs were found to form significantly more adipocytes than both BMPR1A(−) and unenriched cells (**p < 0.01). *p < 0.05. GFP, green fluorescent protein.
Discussion
SVF is a highly heterogeneous cell population and is composed of cells, such as pericytes, endothelial cells, and fibroblasts, in addition to ASCs.10,18 However, ASCs are themselves heterogeneous, being composed of subpopulations with varying capacities for differentiation into specific lineages. A logical approach to the development of ASC-based strategies for the reconstruction of soft tissue defects would thus be to utilize cell populations with enhanced adipogenic differentiation capability. While multiple studies have described pro-osteogenic ASC subpopulations, literature on the existence of proadipogenic subpopulations is limited.6–8 As the BMP signaling pathway is crucial to the process of adipogenic differentiation of mesenchymal precursor cells, we investigated the potential of BMPR1A as a marker for a proadipogenic ASC subpopulation.11,19 While FACS admittedly provides the most precise enrichment of cell populations, MACS has been shown to be less detrimental to cell viability and function, and can be accomplished in a shorter period of time.20 Thus, MACS was chosen as a strategy to obtain a cell population enriched for BMPR1A in this study.
ASCs enriched for the presence of BMPR1A appeared to be more adipogenic in vitro than both the BMPR1A(−) and unenriched cell populations, a fact that was demonstrated by increased PPAR-γ expression with significantly increased FABP4 expression and ORO staining seen in BMPR1A(+) cells, after culture in ADM containing BMP2. Overall, these findings are not surprising given the known functional role of BMP signaling in the lineage commitment of mesenchymal stem cells. BMP2 has specifically been implicated in this process, with concentration-dependent effects: high concentrations promote differentiation into osteoblasts and chondrocytes, whereas low concentrations appear to promote adipogenesis.16 With regard to adipogenic differentiation, BMP2 is known to exert its effects through a complex of type 1 and 2 BMPRs on the cell surface; binding of BMP2 results in phosphorylation and activation of BMPR1A.12,21 Activated BMPR1A goes on to induce two other signaling pathways, one involving the activation of various Smads, beginning with Smad1, and the other culminating in the activation of p38 kinase; Smad signaling eventually functions to increase expression of PPAR-γ, while p38 kinase increases its transcriptional activity.22 While we observed the highest PPAR-γ expression in BMPR1A(+) cells, the fact that this difference was not statistically significant suggests that the latter is likely responsible for the significantly increased terminal adipocytic differentiation seen both in vitro and in vivo in the enriched cells. That is, the increased formation of adipocytes by BMPR1A(+) cells is likely due to increased functioning of the PPAR-γ protein, and thus would not be reflected in levels of PPAR-γ gene expression.
BMP-mediated signaling has been shown to be critical for in vivo adipogenesis. Jin et al. found that mice lacking in Schnurri-2 (Shn2), a protein that interacts with Smad1 to facilitate the expression of PPAR-γ, have significantly reduced adiposity. Notably, Shn2 can only complex with Smad1 after BMP2 stimulation facilitates its entry into the nucleus.16,21 Furthermore, increased BMPR1A expression has been found in overweight and obese individuals in comparison to those with normal body mass indices.23
As the ultimate promise of BMPR1A(+) ASCs for applications in regenerative medicine lies in their adipogenic potential in vivo, we performed transplant experiments using cells transduced with a GFP lentivirus to differentiate them from murine cells. The mouse inguinal fat pad was chosen as the recipient site for these cells as it provides a naturally proadipogenic, nonhypoxic microenvironment. This environment proved suitable for the replication of our in vitro findings: ASCs enriched for BMPR1A expression demonstrated superior adipogenesis in comparison to BMPR1A(−) and unenriched cells. Interestingly, we observed significantly decreased adipogenesis among BMPR1A(−) ASCs, with GFP+ cells visible primarily in perivascular locations, as well as appearing to contribute to fibrous tissue formation (Fig. 4a). This is in keeping with our in vitro data that BMPR1A(−) cells expressed the lowest levels of both PPAR-γ and FABP4 during adipogenic differentiation, and suggests the possibility that the BMPR1A(+) subfraction of ASCs may not only be a proadipogenic subpopulation of cells, but may, in fact, be responsible for the in vivo adipogenesis observed in fat pads receiving unenriched ASCs.
The similarities between BMPR1A(+) and unenriched cells also hold true when cell proliferation is assessed. BMPR1A(−) cells proliferate significantly more rapidly in vitro, reaching higher cell numbers, while BMPR1A(+) and unenriched ASCs expand at a slower rate. This likely speaks of the heterogeneity of such populations, given the observation that BMPR1A(−) cells appear to have a predilection for the formation of fibrous tissue in vivo and are significantly less adipogenic than both BMPR1A(+) and unenriched ASCs, it is not surprising that their proliferation abilities and adaptation to the artificial environment of a cell culture dish might also be different.
The enhanced in vitro and in vivo adipogenic potential of ASCs enriched for the presence of BMPR1A holds great promise for use in cell-based strategies for soft tissue reconstruction. Fat grafting, commonly performed for reconstruction of soft tissue defects, is complicated by resorption rates ranging from 10% to 90% that often necessitate reoperation.24–26 Initially described by Matsumoto et al. in 2006, the addition of supplemental SVF cells/ASCs has been shown to improve fat graft retention.27 However, several unknowns, including the optimum ratio of cells to lipoaspirate for the graft volume desired, remain to be determined before cell-assisted lipotransfer can be consistently used clinically.28,29 While ASC-mediated promotion of fat graft retention has primarily been attributed to paracrine effects, studies suggest that ASCs may also play a critical role in the regeneration of adipocytes within grafts, highlighting their heterogeneity as a cell population.30 As a large proportion of the original adipocytes within a fat graft ultimately die, their replacement is dependent on the adipogenic differentiation of ASCs found locally within the graft or which have been recruited from the surrounding recipient site.31–33 Furthermore, in settings requiring small volume fat transfer, such as during craniofacial reconstruction, an alternative strategy would be to transplant specialized ASC subpopulations capable of de novo adipogenesis into existing fat depots.
De novo adipogenesis as a method for soft tissue reconstruction has been investigated in the past, although primarily in the context of determining the ideal cell delivery mechanism for induction of adipocyte formation. Kimura et al. found that seeding preadipocytes onto a collagen sponge, along with gelatin microspheres loaded with basic fibroblast growth factor, facilitated adipose tissue formation after implantation into immunocompromised mice, while Tsuji et al. performed a similar study using ASCs.5,34 Such studies illustrate the potential for de novo adipogenesis, and suggest a need for future experiments to determine the ideal combination of biomechanical delivery vehicle and cell subpopulation for maximum tissue formation. As evidenced by both our in vitro and in vivo findings, BMPR1A(+) ASCs are promising candidates for such experiments, as they demonstrate enhanced responsiveness to adipogenic cues, likely due to the functional role of BMPR1A in facilitating adipocyte formation. Thus, enrichment for BMPR1A(+) cells facilitates isolation of a population of ASCs with an increased potential for de novo adipogenesis, with the clinical potential to allow for relatively controlled adipose tissue formation in localized, anatomically relevant areas.
Acknowledgments
M.T.L. was supported by NIH grants U01 HL099776, R01 DE021683-01, RC2 DE020771, the Oak Foundation, the Gunn/Olivier Fund, and the Hagey Laboratory for Pediatric Regenerative Medicine. D.C.W. was supported by NIH grant 1K08DE024269, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award. The project described was also supported in part by Award Number S10RR017959 from the National Center for Research Resources (NCRR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or the National Institutes of Health.
Disclosure Statement
No competing financial interests exist for any of the authors of this study.
References
- 1.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., and Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13, 4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao W., Qiao X., Ma S., and Cui L. Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1alpha. J Cell Mol Med 15, 2575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollenbeck S.T., Senghaas A., Komatsu I., Zhang Y., Erdmann D., and Klitzman B. Tissue engraftment of hypoxic-preconditioned adipose-derived stem cells improves flap viability. Wound Repair Regen 20, 872, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Kolle S.F., Fischer-Nielsen A., Mathiasen A.B., Elberg J.J., Oliveri R.S., Glovinski P.V., Kastrup J., Kirchhoff M., Rasmussen B.S., Talman M.L., Thomsen C., Dickmeiss E., and Drzewiecki K.T. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 382, 1113, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Tsuji W., Inamoto T., Yamashiro H., Ueno T., Kato H., Kimura Y., Tabata Y., and Toi M. Adipogenesis induced by human adipose tissue-derived stem cells. Tissue Eng Part A 15, 83, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Levi B., Wan D.C., Glotzbach J.P., Hyun J., Januszyk M., Montoro D., Sorkin M., James A.W., Nelson E.R., Li S., Quarto N., Lee M., Gurtner G.C., and Longaker M.T. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem 286, 39497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung M.T., Liu C., Hyun J.S., Lo D.D., Montoro D.T., Hasegawa M., Li S., Sorkin M., Rennert R., Keeney M., Yang F., Quarto N., Longaker M.T., and Wan D.C. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19, 989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArdle A., Chung M.T., Paik K.J., Duldulao C., Chan C., Rennert R., Walmsley G.G., Senarath-Yapa K., Hu M., Seo E., Lee M., Wan D.C., and Longaker M.T. Positive selection for bone morphogenetic protein receptor type-IB promotes differentiation and specification of human adipose-derived stromal cells toward an osteogenic lineage. Tissue Eng Part A 20, 3031, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., Zimmerlin L., Marra K.G., Donnenberg V.S., Donnenberg A.D., and Rubin J.P. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstruct Surg 128, 663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., and Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D., Ji X., Harris M.A., Feng J.Q., Karsenty G., Celeste A.J., Rosen V., Mundy G.R., and Harris S.E. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142, 295, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H., Song T.J., Li X., Hu L., He Q., Liu M., Lane M.D., and Tang Q.Q. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 106, 12670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S.Y., Lee J.H., Kim J.Y., Bae Y.C., Suh K.T., and Jung J.S. BMP2 increases adipogenic differentiation in the presence of dexamethasone, which is inhibited by the treatment of TNF-alpha in human adipose tissue-derived stromal cells. Cell Physiol Biochem 34, 1339, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Zielins E.R., Tevlin R., Hu M.S., Chung M.T., McArdle A., Paik K.J., Atashroo D., Duldulao C.R., Luan A., Senarath-Yapa K., Walmsley G.G., Wearda T., Longaker M.T., and Wan D.C. Isolation and enrichment of human adipose-derived stromal cells for enhanced osteogenesis. J Vis Exp 95, 52181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry R., Church C.D., Gericke M.T., Jeffery E., Colman L., and Rodeheffer M.S. Imaging of adipose tissue. Methods Enzymol 537, 47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen E.D., and MacDougald O.A. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7, 885, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Jang M.K., and Jung M.H. ATF3 inhibits PPARgamma-stimulated transactivation in adipocyte cells. Biochem Biophys Res Commun 456, 80, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Zimmerlin L., Donnenberg V.S., Pfeifer M.E., Meyer E.M., Peault B., Rubin J.P., and Donnenberg A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77, 22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gesta S., Tseng Y.H., and Kahn C.R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Li Q., Zhang X., Peng Y., Chai H., Xu Y., Wei J., Ren X., Wang X., Liu W., Chen M., and Huang D. Comparison of the sorting efficiency and influence on cell function between the sterile flow cytometry and immunomagnetic bead purification methods. Prep Biochem Biotechnol 43, 197, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Jin W., Takagi T., Kanesashi S.N., Kurahashi T., Nomura T., Harada J., and Ishii S. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell 10, 461, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hata K., Nishimura R., Ikeda F., Yamashita K., Matsubara T., Nokubi T., and Yoneda T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 14, 545, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottcher Y., Unbehauen H., Kloting N., Ruschke K., Korner A., Schleinitz D., Tonjes A., Enigk B., Wolf S., Dietrich K., Koriath M., Scholz G.H., Tseng Y.H., Dietrich A., Schon M.R., Kiess W., Stumvoll M., Bluher M., and Kovacs P. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 58, 2119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herold C., Ueberreiter K., Busche M.N., and Vogt P.M. Autologous fat transplantation: volumetric tools for estimation of volume survival. A systematic review. Aesthet Plast Surg 37, 380, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Ross R.J., Shayan R., Mutimer K.L., and Ashton M.W. Autologous fat grafting: current state of the art and critical review. Ann Plast Surg 73, 352, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Wetterau M., Szpalski C., Hazen A., and Warren S.M. Autologous fat grafting and facial reconstruction. J Craniofac Surg 23, 315, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto D., Sato K., Gonda K., Takaki Y., Shigeura T., Sato T., Aiba-Kojima E., Iizuka F., Inoue K., Suga H., and Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 12, 3375, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Paik K.J., Zielins E.R., Atashroo D.A., Maan Z.N., Duscher D., Luan A., Walmsley G.G., Momeni A., Vistnes S., Gurtner G.C., Longaker M.T., and Wan D.C. Studies in fat grafting: Part V. Cell-assisted lipotransfer to enhance fat graft retention is dose dependent. Plast Reconstruct Surg 136, 67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielins E.R., Luan A., Brett E.A., Longaker M.T., and Wan D.C. Therapeutic applications of human adipose-derived stromal cells for soft tissue reconstruction. Discov Med 19, 245, 2015 [PubMed] [Google Scholar]
- 30.Garza R.M., Paik K.J., Chung M.T., Duscher D., Gurtner G.C., Longaker M.T., and Wan D.C. Studies in fat grafting: Part III. Fat grafting irradiated tissue—improved skin quality and decreased fat graft retention. Plast Reconstruct Surg 134, 249, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato H., Mineda K., Eto H., Doi K., Kuno S., Kinoshita K., Kanayama K., and Yoshimura K. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstruct Surg 133, 303e, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Eto H., Kato H., Suga H., Aoi N., Doi K., Kuno S., and Yoshimura K. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstruct Surg 129, 1081, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Dong Z., Peng Z., Chang Q., Zhan W., Zeng Z., Zhang S., and Lu F. The angiogenic and adipogenic modes of adipose tissue after free fat grafting. Plast Reconstruct Surg 135, 556e, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Kimura Y., Ozeki M., Inamoto T., and Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials 24, 2513, 2003 [DOI] [PubMed] [Google Scholar]