Fig. 6.
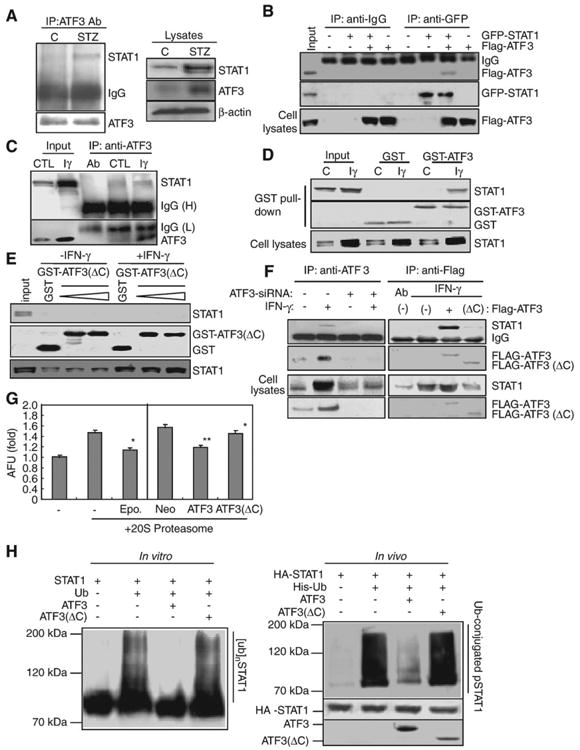
ATF3 increased STAT1 stability by inhibiting Ub/proteasome-dependent STAT1 degradation in hepatocytes. (A) Immunoprecipitation for ATF3 in STZ-injected mice. (B) Cells were transfected with GFP-STAT1 and Flag-ATF3, then immunoprecipitated with anti-GFP. (C) In vivo binding assay. After treatment of IFN-γ, immunocaptured ATF3 was directly interacted with STAT1. (D) GST pull-down assay by using purified GST or GST-ATF3. (E) GST pull-down assay by using purified GST or GST-ATF3(ΔC). (F) Immunocaptured Flag-ATF3, but not ATF3 siRNA (left) or Flag-ATF3(ΔC), directly interacts with STAT1 induced by IFN-γ. (G) 20S proteasome assay. *P<0.01, **P<0.05 in comparison with corresponding control group. (H) In vitro (left) and in vivo (right) ubiquitination assay. GST-STAT1 protein was preincubated with 250 ng of the full-length or the deleted mutant ATF3 protein and then subjected to Western blotting for ubiquitinated STAT1 using anti-polyubiquitin antibody (FK-1) (left). Ubiquitinated products were recovered on nickel-agarose beads and separated by SDS-PAGE, followed by immunoblotting with anti-HA antibody (right).
