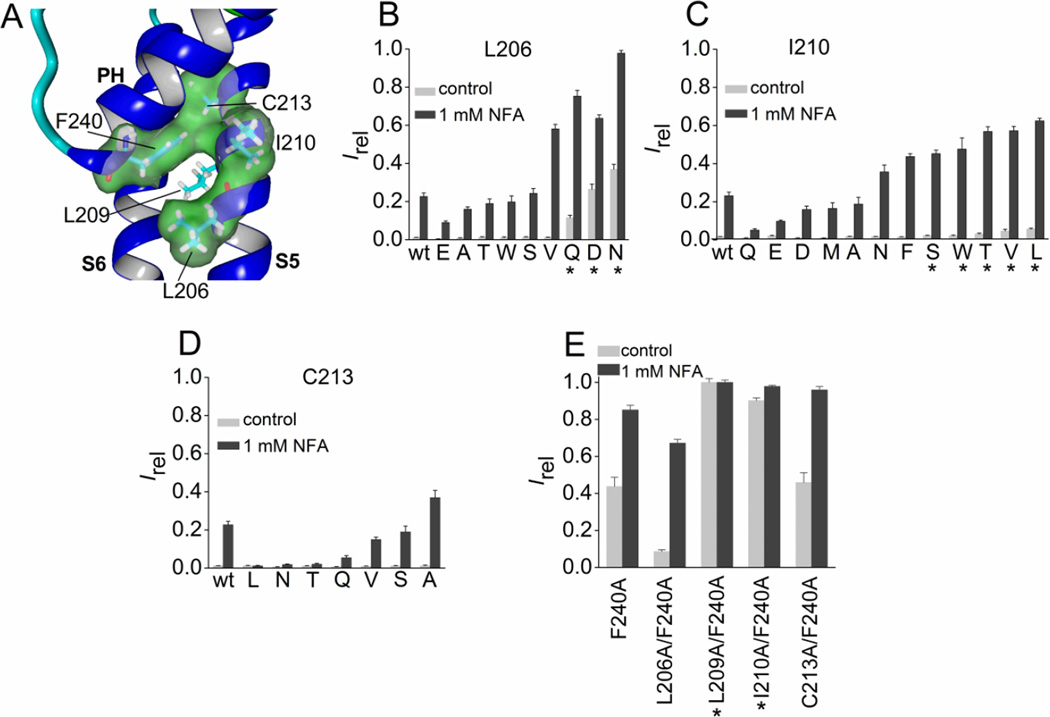
Fig. 5.
Mutation analysis of three residues in the S5 segment of Slo2.1 predicted to be in close proximity to Phe-240. (A) Slo2.2-based homology model of a Slo2.1 subunit, highlighting the close proximity of three residues in the S5 segment to Phe-240 located at the base of the pore helix. For clarity, the molecular surface for Leu-209 in S5 is not shown. (B–D) Irel measured at 0 mV in the absence (control) or presence of 1 mM NFA for wt channels (n = 14), Leu-206 mutant channels (B, n = 5–8), Ile-210 mutant channels (C, n = 5–10) and Cys-213 mutant channels (D, n = 5–8). (E) Combined Ala substitutions of Phe-240 and a single nearby S5 residue. Irel was measured at 0 mV in the absence (control) or presence of 1 mM NFA for Phe-240 Slo2.1 channels containing an additional Ala substitution of the indicated S5 residue (n = 5–8). For panels B–E, amino acid substitutions of the indicated native residue are indicated by single letter code and are ordered by increasing Irel. *, indicates an increase (p < 0.01) in both measures of Irel compared to wt channels. Error bars indicate + S.E.