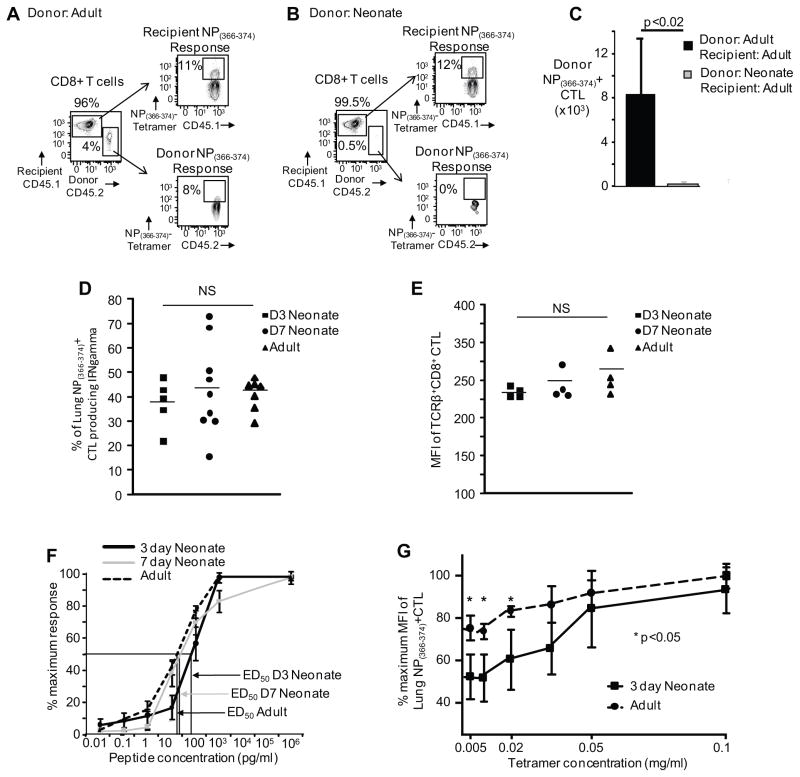
Figure 6. Expansion of neonatal non-transgenic CD8+ T-cells after adoptive transfer into adult mice is diminished as compared to adult non-transgenic CD8+ T cells.
Splenocytes from neonatal and adult mice with matched numbers of CD8+ lymphocytes were adoptively transferred into adults, mice were infected with influenza virus and the expansion of donor CD8+ cells were analyzed on day 10. Representative flow cytometry plots from pulmonary lymphocytes depict percentage of donor NP(366–374)-specific CTLs from adult (A) and neonatal mice (B) versus the recipient’s endogenous response. (C) Pooled data showing absolute numbers of the donor NP(366–374)-specific CTLs from the lungs 10 days post-infection (n= 4–6 mice per group, four independent experiments). (D) Similar IFNγ production after NP(366–374)-peptide stimulation. Percentage of NP(366–374)-specific CTLs (based on MHC I tetramer staining) producing IFNγ is similar among 3-day old, 7-day old and adult mice. (E) Similar MFI of TCRβ+CD8+ CTL among 3-day old, 7-day old and adult mice. (F) Reduced binding affinity of the 3-day old TCR. NP(366–374)-peptide responses (0.01 pg – 1 μg) CTLs 14 days post-infection is depicted as percentage maximum response determined by IFNγ production (n= 4–9 mice per group, 2–3 independent experiments). Arrows indicate ED50 for the different groups. (G) Tetramer titration assay. Equal numbers of adult CD8+ CTL at day 10 post infection and 3-day old CD8+ CTL at day 14 post infection were co-stained with decreasing concentrations of NP(366–374) loaded MHC class I Db tetramer (n= 4 mice per group). Statistical analysis was performed using the Student’s t-test for unpaired samples.