Fig. 4. The effect of mutations in the putative N-glycosylation sites of hTPPT on the migration pattern of the hTPPT protein and its transport function.
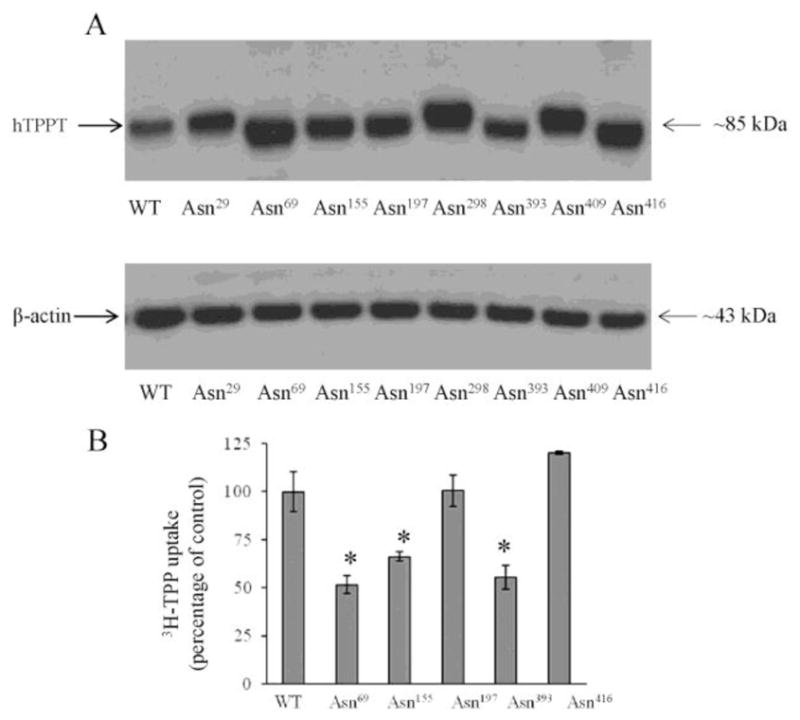
A, Western blot analysis of cell lysates prepared from ARPE19 cells transiently transfected with the wild-type (WT) or the mutant forms of hTPPT (FLAG - tagged everywhere) was performed using monoclonal anti-FLAG antibody (upper panel) or monoclonal anti-β-actin antibody (lower panel). The images are representatives of two independent experiments with similar results. B, Effect of mutations in N-linked glycosylation sites of hTPPT on carrier-mediated TPP uptake. ARPE19 cells transiently expressing mutants (or control, wild-type hTPPT, WT) were used for TPP uptake measurements. [3H]-TPP (0.3 μM) was added to the incubation medium (KR buffer of pH 7.4) at the onset of incubation, and uptake was measured after 5min of incubation. Carrier-mediated TPP uptake by the induced system was calculated as described in “Materials and Methods”. Data are mean ± S.E. of six to eight separate uptake determinations. *p < 0.01.
