Abstract
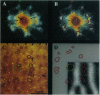
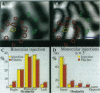
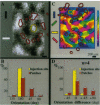
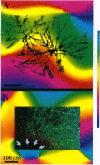
In primate primary visual cortex, neurons sharing similar response properties are clustered together forming functional domains that appear as a mosaic of patches or bands, often traversing the entire cortical depth from the pia to the white matter. Similarly, each cortical site connects laterally through an extensive network of intrinsic projections that are organized in multiple clusters (patches) and reach distances of up to a few millimeters. The relationship between the functional domains and these laterally connected patches has remained a controversial issue despite intensive research efforts. To investigate this relationship, we obtained high-resolution functional maps of the cortical architecture by in vivo optical imaging. Subsequently, extracellular injections of the sensitive anterograde tracer biocytin were targeted into selected functional domains. Within the ocular dominance system, we found that long-range intrinsic connections tended to link the monocular regions of same-eye ocular dominance columns. Furthermore, we discovered that binocular domains formed a separate set of connections in area V1; binocular regions were selectively connected among themselves but were not connected to strictly monocular regions, suggesting that they constitute a distinct columnar system. In the other subsystem subserving orientation preference, patches of intrinsic connections tended to link domains sharing similar orientation preferences. Analyses of the precision of these connections indicated that in both functional subsystems, < 15% of the connections were between domains having orthogonal response properties. However, their selectivity was limited; approximately 30% +/- 10% of the interconnected patches contained neurons exhibiting orientation tuning that differed from those found at the injection sites by at least 45 degrees. At short range (up to 400 microns from the injection site), this casual trend seemed markedly accentuated; the local, synaptic-rich axonal and dendritic arbors crossed freely through columns of diverse functional properties. These complex sets of connections can endow cortical neurons with a rich diversity of response properties and broad tuning.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir Y., Harel M., Malach R. Cortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual cortex. J Comp Neurol. 1993 Aug 1;334(1):19–46. doi: 10.1002/cne.903340103. [DOI] [PubMed] [Google Scholar]
- Barlow H. B. The Ferrier Lecture, 1980. Critical limiting factors in the design of the eye and visual cortex. Proc R Soc Lond B Biol Sci. 1981 May 7;212(1186):1–34. doi: 10.1098/rspb.1981.0022. [DOI] [PubMed] [Google Scholar]
- Bartfeld E., Grinvald A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11905–11909. doi: 10.1073/pnas.89.24.11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G. Orientation selectivity, preference, and continuity in monkey striate cortex. J Neurosci. 1992 Aug;12(8):3139–3161. doi: 10.1523/JNEUROSCI.12-08-03139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986 Jun 5;321(6070):579–585. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T., Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991 Oct 3;353(6343):429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- Fiorani Júnior M., Rosa M. G., Gattass R., Rocha-Miranda C. E. Dynamic surrounds of receptive fields in primate striate cortex: a physiological basis for perceptual completion? Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8547–8551. doi: 10.1073/pnas.89.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hubel D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981 Aug 20;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Uniformity of monkey striate cortex: a parallel relationship between field size, scatter, and magnification factor. J Comp Neurol. 1974 Dec 1;158(3):295–305. doi: 10.1002/cne.901580305. [DOI] [PubMed] [Google Scholar]
- King M. A., Louis P. M., Hunter B. E., Walker D. W. Biocytin: a versatile anterograde neuroanatomical tract-tracing alternative. Brain Res. 1989 Sep 18;497(2):361–367. doi: 10.1016/0006-8993(89)90281-3. [DOI] [PubMed] [Google Scholar]
- LeVay S. Patchy intrinsic projections in visual cortex, area 18, of the cat: morphological and immunocytochemical evidence for an excitatory function. J Comp Neurol. 1988 Mar 8;269(2):265–274. doi: 10.1002/cne.902690210. [DOI] [PubMed] [Google Scholar]
- LeVay S. The patchy intrinsic projections of visual cortex. Prog Brain Res. 1988;75:147–161. doi: 10.1016/s0079-6123(08)60474-4. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Specificity of intrinsic connections in primate primary visual cortex. J Neurosci. 1984 Nov;4(11):2830–2835. doi: 10.1523/JNEUROSCI.04-11-02830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwel S., Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992 Jan 10;255(5041):209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Malach R. Dendritic sampling across processing streams in monkey striate cortex. J Comp Neurol. 1992 Jan 15;315(3):303–312. doi: 10.1002/cne.903150306. [DOI] [PubMed] [Google Scholar]
- Matsubara J., Cynader M., Swindale N. V., Stryker M. P. Intrinsic projections within visual cortex: evidence for orientation-specific local connections. Proc Natl Acad Sci U S A. 1985 Feb;82(3):935–939. doi: 10.1073/pnas.82.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A., Hornung J. P., Gilbert C. D., Wiesel T. N. Patterns of synaptic input to layer 4 of cat striate cortex. J Neurosci. 1984 Dec;4(12):3021–3033. doi: 10.1523/JNEUROSCI.04-12-03021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983 May 20;216(3):303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Widespread periodic intrinsic connections in the tree shrew visual cortex. Science. 1982 Mar 19;215(4539):1532–1534. doi: 10.1126/science.7063863. [DOI] [PubMed] [Google Scholar]
- Swindale N. V., Matsubara J. A., Cynader M. S. Surface organization of orientation and direction selectivity in cat area 18. J Neurosci. 1987 May;7(5):1414–1427. doi: 10.1523/JNEUROSCI.07-05-01414.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., Hamilton S. L., De Valois R. L., Switkes E. Functional anatomy of macaque striate cortex. III. Color. J Neurosci. 1988 May;8(5):1569–1593. doi: 10.1523/JNEUROSCI.08-05-01569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S. Two methods for flat-mounting cortical tissue. J Neurosci Methods. 1985 Nov-Dec;15(3):177–190. doi: 10.1016/0165-0270(85)90097-4. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Frostig R. D., Lieke E. E., Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990 Jul 27;249(4967):417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D. The organization of chromatic and spatial interactions in the primate striate cortex. J Neurosci. 1988 May;8(5):1712–1727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C., Newsome W. T., Maunsell J. H. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24(5):429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]