Abstract
Objective. We evaluated synergistic efficacy and safety of combined topical application of Botulinum Toxin Type A (BTX-A) with fractional CO2 laser for facial rejuvenation. Methods. Twenty female subjects were included for this split-face comparative study. One side of each subject's cheek was treated with fractional CO2 plus saline solution, and the other side was treated with fractional CO2 laser plus topical application of BTX-A. Patients received one session of treatment and evaluations were done at baseline, one, four, and twelve weeks after treatment. The outcome assessments included subjective satisfaction scale; blinded clinical assessment; and the biophysical parameters of roughness, elasticity, skin hydration, transepidermal water loss (TEWL), and the erythema and melanin index. Results. BTX-A combined with fractional CO2 laser sides showed higher physician's global assessment score, subject satisfaction score, roughness, skin hydration, and skin elasticity compared to that of fractional CO2 plus saline solution side at 12 weeks after treatment. TEWL and erythema and melanin index showed no significant differences between two sides at baseline, one, four, and twelve weeks after treatment. Conclusion. Topical application of BTX-A could enhance the rejuvenation effect of fractional CO2 laser.
1. Introduction
Fractional CO2 laser has now been considered as the gold standard for skin rejuvenation [1]. Nevertheless, when it is used for facial rejuvenation, it often requires multiple therapy sessions which will be a kind of psychological and economic burden for the patients. Besides that, fractional CO2 laser is still reported to have a variety of complications, such as postinflammatory hyperpigmentation, prolonged erythema, skin swelling, infection, and scarring [2, 3]. How to reduce adverse reactions of fractional CO2 laser, while enhancing its rejuvenation effect, has become one hot topic in dermatological research field.
Botulinum Toxin Type A (BTX-A) is one of seven neurotoxins produced by Clostridium botulinum. BTX-A can result in muscle fiber atrophy and subsequent clinical flaccid paralysis [4]. Consequently, its cosmetic use by subdermal injections in treating wrinkles induced by muscle hyperactivity is widespread [5]. Some physicians have observed a face-lifting effect after intradermal injection of BTX-A to the mid and lower face [6, 7]; however the underlying mechanisms still remain unclear. In 2012, Oh et al. studied the in vitro effects of BTX-A on normal fibroblasts and found that BTX-A has a significant effect in increasing the level of collagen production and downregulating its degradation [8]. In 2014, our research group further confirmed the direct antiphotoaging potential of BTX-A in UVB-induced premature senescence of human dermal fibroblasts in vitro through decreasing senescence-related proteins [9]. Whether BTX-A shows its direct skin rejuvenation effects has not been studied in vivo as yet.
Recent studies have also reported the transepidermal delivery of drugs, stem cells secreted factors, and even bone marrow mesenchymal stem cells using a fractional ablative laser [10–12]. According to the results of previous literatures, we can speculate that BTX-A can pass through the micropores created by fractional CO2 laser and penetrate into the dermis, thereby executing various biological effects. In present 12-week clinical study, BTX-A solution was topically administrated following fractional CO2 laser; we then focused on the effects of BTX-A on the efficacy and adverse effects after fractional CO2 laser.
2. Material and Methods
2.1. Subjects
This was a single-center prospective pilot study. All subjects provided written informed consent, and this study was approved by the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University. Twenty female subjects with Fitzpatrick phototypes III and IV were enrolled in the study after signing an informed consent form. Patients were aged 21 to 53 (mean age 35.6). Exclusion criteria were keloids and hypertrophic scars, cancer lesions, warts or skin infections in the area to be treated, viral herpes infections during the previous 6 months, collagen disease, and autoimmune disease. Individuals who had taken systemic isotretinoin, BTX-A injection, or any facial rejuvenation procedures during the previous 12 months, had used nonreabsorbable fillers, were undergoing treatment with antineoplastics, corticosteroids, or anticoagulants, or were diabetic, pregnant, or breastfeeding were excluded.
2.2. Treatment Protocol
A topical anesthetic cream (2.5% lidocaine and 2.5% prilocaine; Tsinghua Ziguang Co., Beijing, China) was applied for 30 minutes before treatment and then completely removed. A fractional CO2 laser (Acupulse, Lumenis, Inc., Santa Clara, CA, USA) was used for the fractional laser treatment. We treat both cheeks' area of subjects with DeepFX microscanner handpiece of the fractional ultrapulsed CO2 laser. The laser parameters were set: single pass, 0.12 mm spot size, pulse energy 10 mJ, density 5%, pulse size 10 × 10 mm, and repetition rate 300 Hz. The peak power and beam width of the fractional ultrapulsed CO2 laser are 200 watt and 50–80 μs, respectively. Ten milliliters of BTX-A solution (diluted in saline solution, concentration at 5 μ/mL) was topically applied onto the fractional laser-treated sites of one randomly selected facial side for one hour, while saline solution was applied to fractional laser-treated sites of the another face side for one hour. The outcome assessments included the subjective satisfaction scale, improvement score according to blinded investigators (using standardized photography), and biophysical measurements. Measurements were conducted at baseline, one, four, and twelve weeks after the treatment. Subjects were permitted to apply their usual skin care products throughout the study. It was requested that subjects do not alter their usual skincare routine during the study period.
2.3. Outcome Assessment
2.3.1. Subjective Satisfaction Scale
Subjects completed a self-assessment questionnaire and rated their improvement on a scale from 0 (aggravated) to 4 (much improved). After treatment, subjects were asked to grade their intraprocedure pain on a 10 cm visual analog scale (VAS), with the end points designated as 0 (no pain) and 10 (the worst pain imaginable). The investigator subjectively graded edema after treatment on a scale from 0 to 4 (0 = absent, 1 = trace, 2 = slight, 3 = moderate, and 4 = prominent). The duration of erythema and crusting was investigated through interviews. Any adverse events and complications were recorded at the time of each treatment and at the follow-up visit.
2.4. Blinded Clinical Assessment
Standardized photographs were obtained at baseline and 1 month after the last treatment. Standardized close-up photographs were taken using a Visia photo stand (Canfield Imaging Systems, Fairfield, NJ) mounted with a high resolution digital camera (Canon EOS-40D, Canon Corp., Tokyo, Japan). Two dermatologists who were blinded to subject treatment group evaluated the serial photographs independently and performed clinical assessments on fine wrinkles, coarse wrinkles, roughness, mottled hyperpigmentation, laxity, and skin tone using a well-established grading scale of 0 ≤ 25% (minimal), 1 = 26–50% (fair), 2 = 51–75% (good), 3 = 75–90% (excellent), and 4 = 91–100% (clear) improvement. Average improvement scores were calculated as the mean of the grading scales of all categories.
2.5. Biophysical Evaluations
The parameters assessed were in vivo erythema, melanin, transepidermal water loss (TEWL), elasticity, skin surface roughness, and hydration, which were measured with respective probes (Courage and Khazaka Electronic GmbH, Cologne, Germany). All measurements were taken after subjects had undergone an acclimatization period of at least 20 minutes in an air-conditioned room under standardized conditions (22–25°C, 50% humidity). Each measurement was performed on the left and right cheeks of each subject.
2.6. Statistical Analysis
The results were analyzed with the paired t-test using SPSS 15.0 software (SPSS, Inc., Chicago, IL). P < 0.05 was considered to be significant.
3. Results
3.1. Objective Clinical Assessment
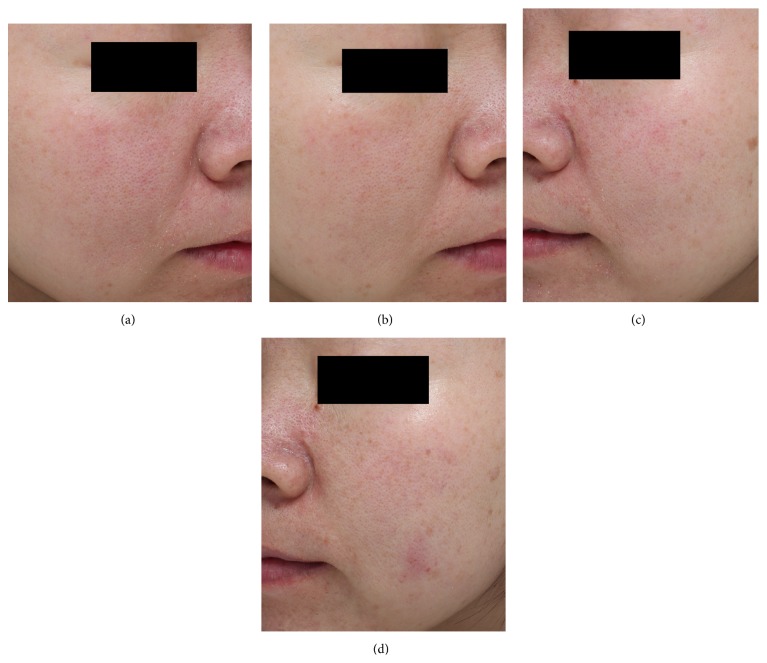
Figure 1 shows representative clinical manifestations of both treatment sides taken at indicated time points. During the evaluation periods, two blinded evaluators scores increased successively in both sides. Twelve weeks after the treatment, BTX-A side objective clinical assessment score value was 3.40 ± 0.42 which was significantly higher than that of saline solution side (2.70 ± 0.43) (P < 0.05).
Figure 1.
Representative clinical photographs: (a, c) baseline, (b) 12 weeks after the BTX-A + fractionated CO2 laser treatment, and (d) 12 weeks after the saline solution + fractionated CO2 laser treatment.
3.2. Subjective Satisfaction Scale
During the evaluation periods, the subjective satisfaction scores in both sides increased successively. Twelve weeks after the treatment, BTX-A side subjective satisfaction score value was 3.40 ± 0.53 which was significantly higher than that of saline solution side (2.70 ± 0.47) (P < 0.05).
3.3. Biophysical Analysis
3.3.1. Erythema Index (EI) and Melanin Index (MI)
As shown in Figures 2(a) and 2(b), during the study, the average value of EI and MI in both sides achieved the peak value at one week after the treatment, while decreasing to nearly baseline four weeks after treatment. However, the differences between the two sides were not statistically significant at any given point of time after the treatment.
Figure 2.
Skin measurements by biophysical evaluation methods were taken at baseline, 1 week, 4 weeks, and 12 weeks after treatment on both sides. (a) Objective measurement of erythema index, (b) melanin index, (c) overall elasticity (R2), (d) hydration, (e) glossy, and (f) TEWL. ∗ P < 0.05 compared with control side at same time point; # P < 0.05 compared with baseline.
3.4. Elasticity
As shown in Figure 2(c), one week after the treatment, the skin elasticity of both sides was higher than that prior to the treatment, then decreasing to nearly baseline four weeks after the treatment. Twelve weeks after the treatment, the skin elasticity in BTX-A side increased and was higher than that of baseline; however, it remained nearly unchanged in saline solution treated side and the difference of elasticity between the two sides was statistically significant (P > 0.05).
3.5. TEWL
TEWL was as one of the noninvasive indices to evaluate the skin barrier integrity or function. As shown in Figure 2(f), the value of TEWL increased at one week after the treatment and then rapidly decreased to baseline at four weeks after the treatment. In BTX-A side, the average values even showed a lower level than baseline and are statistically significantly lower than that of saline solution side at twelve weeks after treatment (P < 0.05).
3.6. Hydration
Hydration content was also considered as one of the indices to evaluate the skin barrier. As shown in Figure 2(d), hydration increased from 56.08 ± 9.59 to 67.76 ± 3.38 in BTX-A side. The hydration values in BTX-A side shown were significantly higher than that of saline solution side at twelve weeks after treatment (P < 0.05).
3.7. Skin Surface Roughness
As shown in Figure 2(e), skin surface roughness increased significantly (from 8.53 ± 1.0 to 9.13 ± 1.26) in saline solution side and more significantly increased in BTX-A side (from 8.61 ± 1.33 to 10.79 ± 1.29). The differences between the two sides were noted at twelve weeks after treatment (P < 0.05).
3.8. Comparison of Adverse Reactions
No serious or persistent side effects occurred during the course of study, and none of the subjects withdrew from the study because of any adverse event. No hypopigmentation or hypertrophic scarring was observed in any subject throughout the study period. As shown in Table 1, there was no significant difference of pain score, edema score, duration of crust, and duration of erythema between the two sides.
Table 1.
Comparison of adverse events on both sides after treatment ( ).
| Group | Pain score | Edema score | Duration of incrustation (d) | Duration of erythema (d) |
|---|---|---|---|---|
| BTX-A treatment | 5.03 ± 0.81 | 1.73 ± 0.58 | 7.43 ± 2.27 | 20.29 ± 1.75 |
| Saline solution treatment | 4.95 ± 1.03 | 1.97 ± 0.72 | 7.23 ± 2.64 | 20.45 ± 1.33 |
Note. No significant differences were observed on both cheeks (P > 0.05).
4. Discussion
Regarding erythema and melanin index, there are no significant differences between BTX-A side and saline solution control side up to 12 weeks after laser treatment in all subjects. Several reports demonstrated that BTX-A can be used for the treatment of facial erythema and flushing [13–15]. The clinical results were substantiated with corresponding decreases in cutaneous blood flow, as measured using laser Doppler flowmetry [16]. Although BTX-A cannot reduce erythema index after laser treatment, it at least did not induce excessive angiogenesis as shown in the present study. Besides that, there are not any significant differences between the two sides in regard to the pain score, edema score, duration of crusting, and duration of erythema, which verify that adjuvant BTX-A treatment may not help promote the recovery of laser-damaged skin and decrease downtime.
Although an increase in the level of TEWL and a decrease in the hydration content in the stratum corneum one week after the treatment were the signs of impaired skin barrier function, these two indices in both sides recovered to the baseline levels at four weeks after the treatment. Interestingly, at twelve weeks after the treatment, the TEWL was significantly lower, while hydration was significantly higher in BTX-A side than that of saline solution side. These results suggested that although BTX-A did not have an impact on skin recovery, it improves skin barrier function quality after laser treatment. A recent research indicated that the skin barrier function was enhanced after the injection of a new variety of BTX [17]. Besides that, Oh et al. also found that BTX-A could increase the viability of skin fibroblasts, suggesting its role in promoting cell growth and wound healing [8]. Moreover, some researchers have also proven that BTX-A could accelerate the healing process of several kinds of skin wounds [18–20], suggesting BTX-A may activate the proliferation and migration of human skin cells which are important for skin barrier function maintenance. In one word, our results in the present in vivo study further confirmed that better skin barrier function can be achieved by BTX-A topical treatment.
There have been many studies which have suggested that fractional CO2 laser can significantly improve skin texture and wrinkles [21–23]. Similar to previous reports, the subjective satisfaction scores of skin elasticity and texture were significantly better than control sides. Besides that, we also confirmed by biophysical analysis that the skin elasticity and skin surface roughness in BTX-A sides were also higher than those of control sides 12 weeks after treatment. These results suggested that topical application of BTX-A can significantly enhance the facial rejuvenation effect of fractional CO2 laser. Several in vivo histopathological researches have confirmed that depth of the microthermal zones of fractionated CO2 laser, whose settings are similar to the present study, was limited to the middermis [24–26]. Skin permeation of small-molecule drugs, macromolecules, and nanoparticles mediated by a fractional carbon dioxide laser mostly target the skin dermis [27, 28]. In this consideration, we speculate that BTX-A solution used in the present study may take its action mainly on dermis rather than muscle fibers alone. Besides that, the best facial rejuvenation effect of topical BTX-A appeared 12 weeks after laser treatment which further supports our hypothesis. In our previous study, we found BTX-A has positive effects on photoaged fibroblasts in vitro by increasing collagen production, decreasing collagen degradation, and stimulating cell proliferation via decreasing senescence-related proteins [9]. However, up to date, it remains unclear with which cellular signal pathway BTX-A acts as an antiaging molecule. Its exact functional mechanisms deserve further basic in-depth researches.
One major shortcoming of this study is that it is merely a small sample prospective clinical study. A larger sample randomized controlled clinical trials need to verify the results of this study. To the best of our knowledge, our study is first to report the efficacy and safety of topical BTX-A markedly enhancing the facial rejuvenation effect of fractional CO2 laser. We suggest that topical BTX-A can be used as an adjuvant therapy after fractional CO2 laser. Its exact mechanism of action and how to optimize its clinical effects are still worth further researches.
Acknowledgments
This work was supported by Grants from the China National Natural Science Foundation (81573072 and 81301384) and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Conflict of Interests
The authors report no conflict of interests.
References
- 1.Saedi N., Jalian H. R., Petelin A., Zachary C. Fractionation: past, present, future. Seminars in Cutaneous Medicine and Surgery. 2012;31(2):105–109. doi: 10.1016/j.sder.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Oh B. H., Hwang Y. J., Lee Y. W., Choe Y. B., Ahn K. J. Skin characteristics after fractional photothermolysis. Annals of Dermatology. 2011;23(4):448–454. doi: 10.5021/ad.2011.23.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metelitsa A. I., Alster T. S. Fractionated laser skin resurfacing treatment complications: a review. Dermatologic Surgery. 2010;36(3):299–306. doi: 10.1111/j.1524-4725.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- 4.Fagien S. Botox for the treatment of dynamic and hyperkinetic facial lines and furrows: adjunctive use in facial aesthetic surgery. Plastic and Reconstructive Surgery. 1999;103(2):701–713. doi: 10.1097/00006534-199902000-00055. [DOI] [PubMed] [Google Scholar]
- 5.Said S. Z., Meshkinpour A., Carruthers A., Carruthers J. Botulinum toxin A: its expanding role in dermatology and esthetics. American Journal of Clinical Dermatology. 2003;4(9):609–616. doi: 10.2165/00128071-200304090-00003. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez C. M., Tredwell S. J., Keenan S. P., et al. Treatment of idiopathic clubfoot utilizing botulinum A toxin: a new method and its short-term outcomes. Journal of Pediatric Orthopaedics. 2005;25(2):229–235. doi: 10.1097/01.bpo.0000149861.50400.db. [DOI] [PubMed] [Google Scholar]
- 7.Seyler T. M., Smith B. P., Marker D. R., et al. Botulinum neurotoxin as a therapeutic modality in orthopaedic surgery: more than twenty years of experience. The Journal of Bone & Joint Surgery—American Volume. 2008;90(supplement 4):133–145. doi: 10.2106/jbjs.h.00901. [DOI] [PubMed] [Google Scholar]
- 8.Oh S.-H., Lee Y., Seo Y.-J., et al. The potential effect of botulinum toxin type A on human dermal fibroblasts: an in vitro study. Dermatologic Surgery. 2012;38(10):1689–1694. doi: 10.1111/j.1524-4725.2012.02504.x. [DOI] [PubMed] [Google Scholar]
- 9.Permatasari F., Hu Y.-Y., Zhang J.-A., Zhou B.-R., Luo D. Anti-photoaging potential of Botulinum Toxin Type A in UVB-induced premature senescence of human dermal fibroblasts in vitro through decreasing senescence-related proteins. Journal of Photochemistry and Photobiology B: Biology. 2014;133:115–123. doi: 10.1016/j.jphotobiol.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B.-R., Xu Y., Guo S.-L., et al. The effect of conditioned media of Adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/519126.519126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X., Wang H.-Y., Zhang Y., et al. Adipose-derived stem cells cooperate with fractional carbon dioxide laser in antagonizing photoaging: A potential role of Wnt and β-catenin signaling. Cell and Bioscience. 2014;4, article 24 doi: 10.1186/2045-3701-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlendsson A. M., Anderson R. R., Manstein D., Waibel J. S. Developing technology: ablative fractional lasers enhance topical drug delivery. Dermatologic Surgery. 2014;40(supplement 12):S142–S146. doi: 10.1097/dss.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 13.Park K. Y., Hyun M. Y., Jeong S. Y., Kim B. J., Kim M. N., Hong C. K. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230(4):299–301. doi: 10.1159/000368773. [DOI] [PubMed] [Google Scholar]
- 14.Lin K.-H., Wang S.-J., Fuh J.-L., Chen S.-P. Effectiveness of botulinum toxin A in treatment of refractory erythromelalgia. Journal of the Chinese Medical Association. 2013;76(5):296–298. doi: 10.1016/j.jcma.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Geddoa E., Matar H. E., Paes T. R. F. The use of botulinum toxin-A in the management of neck and anterior chest wall flushing: pilot study. International Journal of Dermatology. 2013;52(12):1547–1550. doi: 10.1111/ijd.12200. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Thirumala P. D., Shah A., et al. Effect of previous botulinum neurotoxin treatment on microvascular decompression for hemifacial spasm. Neurosurgical Focus. 2013;34, article E3 doi: 10.3171/2012.11.focus12373. [DOI] [PubMed] [Google Scholar]
- 17.Streker M., Luebberding S., Krueger N., Harrington L., Kerscher M. Patient-reported outcomes after incobotulinumtoxinA treatment for upper facial wrinkles. Dermatologic Surgery. 2015;41:S29–S38. doi: 10.1097/DSS.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 18.Al-Qattan M. M., Al-Shanawani B. N., Alshomer F. Botulinum toxin type A: implications in wound healing, facial cutaneous scarring, and cleft lip repair. Annals of Saudi Medicine. 2013;33(5):482–488. doi: 10.5144/0256-4947.2013.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassner H. G., Brissett A. E., Otley C. C., et al. Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clinic Proceedings. 2006;81(8):1023–1028. doi: 10.4065/81.8.1023. [DOI] [PubMed] [Google Scholar]
- 20.Ziade M., Domergue S., Batifol D., et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. Journal of Plastic, Reconstructive and Aesthetic Surgery. 2013;66(2):209–214. doi: 10.1016/j.bjps.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Gotkin R. H., Sarnoff D. S., Cannarozzo G., Sadick N. S., Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. Journal of Drugs in Dermatology. 2009;8(2):138–144. [PubMed] [Google Scholar]
- 22.Berlin A. L., Hussain M., Phelps R., Goldberg D. J. A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: a clinical and histopathologic evaluation. Dermatologic Surgery. 2009;35(2):222–228. doi: 10.1111/j.1524-4725.2008.34413.x. [DOI] [PubMed] [Google Scholar]
- 23.Buzina D. Š., Lipozenčić J., Mokos Z. B., Čeović R., Kostović K. Ablative laser resurfacing: is it still the gold standard for facial rejuvenation? Acta Dermatovenerologica Croatica. 2010;18(3):190–194. [PubMed] [Google Scholar]
- 24.Oni G., Robbins D., Bailey S., Brown S. A., Kenkel J. M. An in vivo histopathological comparison of single and double pulsed modes of a fractionated CO2 laser. Lasers in Surgery and Medicine. 2012;44(1):4–10. doi: 10.1002/lsm.21150. [DOI] [PubMed] [Google Scholar]
- 25.Prignano F., Bonciani D., Campolmi P., Cannarozzo G., Bonan P., Lotti T. A study of fractional CO2 laser resurfacing: the best fluences through a clinical, histological, and ultrastructural evaluation. Journal of Cosmetic Dermatology. 2011;10(3):210–216. doi: 10.1111/j.1473-2165.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 26.Shin M.-K., Choi J. H., Ahn S. B., Lee M. H. Histologic comparison of microscopic treatment zones induced by fractional lasers and radiofrequency. Journal of Cosmetic and Laser Therapy. 2014;16(6):317–323. doi: 10.3109/14764172.2014.957216. [DOI] [PubMed] [Google Scholar]
- 27.Haedersdal M., Sakamoto F. H., Farinelli W. A., Doukas A. G., Tam J., Anderson R. R. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL) Lasers in Surgery and Medicine. 2014;46(6):462–469. doi: 10.1002/lsm.22259. [DOI] [PubMed] [Google Scholar]
- 28.Lee W.-R., Shen S.-C., Al-Suwayeh S. A., Yang H.-H., Li Y.-C., Fang J.-Y. Skin permeation of small-molecule drugs, macromolecules, and nanoparticles mediated by a fractional carbon dioxide laser: the role of hair follicles. Pharmaceutical Research. 2013;30(3):792–802. doi: 10.1007/s11095-012-0920-4. [DOI] [PubMed] [Google Scholar]