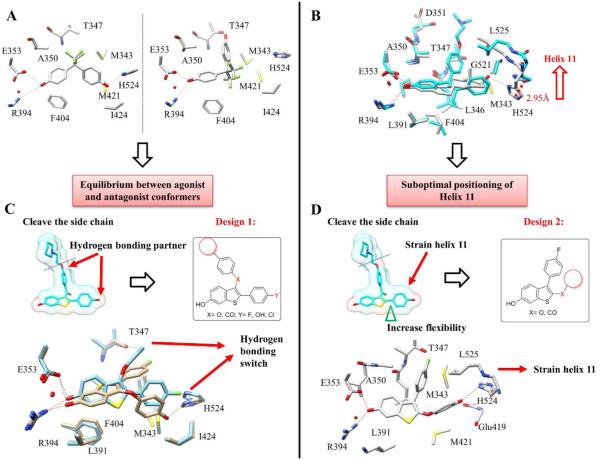
Figure 2.
Design of partial agonists. (A) Crystal structure of BPAF (6) (pdb ID: 3UUA) bound to ER LBD shows two binding modes with hydrogen bonding switch from His-524 to Thr-347, suggestive of alternate binding modes leading to agonist and antagonist conformations that contributes to partial agonist activity. (B) Overlay of E2 (1) and tamoxifen (2) crystal structures (pdb ID: 1GWR and 3ERT) shows the movement of helix 11 (His 524 and Leu 525) that can lead to strain of helix 12, and a partial agonist conformation. (C) Truncation of the side chain of raloxifene and substitution of phenolic OH leads to Design 1. Docking of ligand 19 shows two binding poses with alternate hydrogen bonding. (D) Insertion of a linker and diversifying substituents at C2 of the benzothiophene leads to Design 2. Docking of ligand 30a shows the effect of the elongation of the 2-substituent compatible with a shift of helix 11.