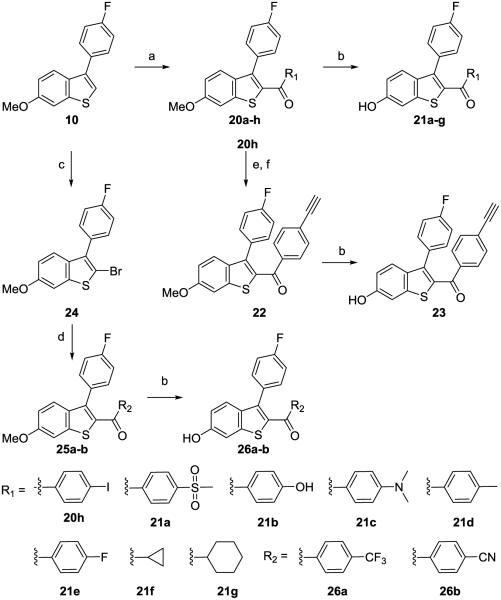
Scheme 3.
Synthesis of 3-(4-fluorophenyl)benzothiophene compounds with a ketone linkera
aReagents and conditions: (a) AlCl3, R1COCl, DCM, rt, 2-48 h; (b) BBr3, DCM, −78 °C to rt, 4-48 h for 21a-e and 26a-b; BF3•SMe2, DCM, 0 °C to rt, 24 h, for 21g and 23; (c) N-bromoacetamide, DCM/EtOH, rt, 89%, 2 h; (d) n-BuLi, R2COCl, THF, −78 °C to rt, 4 h; (e) Et3N, Pd(PPh3)2Cl2, DMF, CuI, ethynyltrimethylsilane, 4 h; (f)TBAF, THF, rt, 1 h, 50% over two steps.