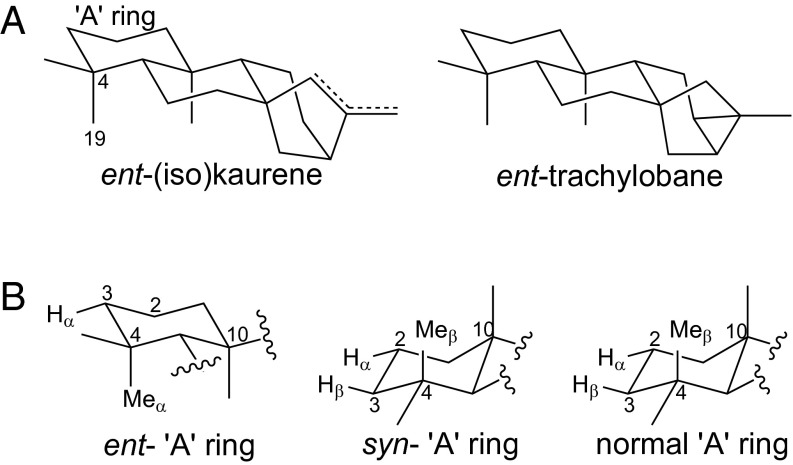
Fig. 5.
Structure–function relationship for the activity exhibited by (A) OsKO2 and (B) AtKO. (A) 3D rendering illustrating the close structural relationship between ent-(iso)kaurene and ent-trachylobane, the only substrates accepted by OsKO2. (B) Configuration of the “A” ring from the three underlying stereoisomers (i.e., of CPP) illustrating targeting of the same two roughly apposed sites with normal and syn-CPP derived LRDs that may be related to their similar configuration in this region. Previous work has demonstrated that other non-KO CYP701A subfamily members (i.e., from rice) target the C3α position with ent- and syn-CPP-derived LRDs (9, 10).