Significance
Virion proteomics represents a powerful and unbiased approach to gain insights into the process of virus assembly and the host factors important for infection. For hepatitis C virus (HCV), it is known that infectious virions are assembled alongside endogenous lipoproteins to give rise to a chimeric lipoviral particle. Host-derived apolipoproteins coat the exterior of circulating particles, making for a veiled pathogen. The protein composition of HCV remains, however, undefined. Here, using MS, we identified novel viral and cellular proteins specifically associated with HCV virions and discovered an essential interaction between the capsid protein and the nucleoporin Nup98 that is required for virion biogenesis.
Keywords: enveloped virus, Flaviviridae, virion proteomics, virus–host interactions, nucleoporin
Abstract
Hepatitis C virus (HCV) is a unique enveloped virus that assembles as a hybrid lipoviral particle by tightly interacting with host lipoproteins. As a result, HCV virions display a characteristic low buoyant density and a deceiving coat, with host-derived apolipoproteins masking viral epitopes. We previously described methods to produce high-titer preparations of HCV particles with tagged envelope glycoproteins that enabled ultrastructural analysis of affinity-purified virions. Here, we performed proteomics studies of HCV isolated from culture media of infected hepatoma cells to define viral and host-encoded proteins associated with mature virions. Using two different affinity purification protocols, we detected four viral and 46 human cellular proteins specifically copurifying with extracellular HCV virions. We determined the C terminus of the mature capsid protein and reproducibly detected low levels of the viral nonstructural protein, NS3. Functional characterization of virion-associated host factors by RNAi identified cellular proteins with either proviral or antiviral roles. In particular, we discovered a novel interaction between HCV capsid protein and the nucleoporin Nup98 at cytosolic lipid droplets that is important for HCV propagation. These results provide the first comprehensive view to our knowledge of the protein composition of HCV and new insights into the complex virus–host interactions underlying HCV infection.
Compositional studies of virions provide powerful clues for understanding the functions of viral proteins; assembly and entry pathways; and, more broadly, mechanisms of virus–host interactions. Increasingly sensitive MS techniques have enabled the detection of viral and cellular proteins that are incorporated in virions even at very low levels (1).
Hepatitis C virus (HCV) is a positive-sense ssRNA virus of the Flaviviridae family. This bloodborne pathogen causes chronic liver infection that develops into cirrhosis and hepatocellular carcinoma and is the leading indication for liver transplantation. Over 185 million people are chronically infected with HCV (2). Despite great advances in the ability to study this virus in vitro, significant gaps remain in our understanding of the infectious particle and the virus–host interactions required for HCV propagation.
The protein composition of HCV is not known. Although the capsid protein, Core, and the E1 and E2 envelope glycoproteins are thought to be the major constituents of the virion, it remains to be determined if nonstructural viral proteins are packaged as well. A growing body of literature suggests that cellular proteins are important components of HCV. Indeed, this virus closely associates with LDL and very-LDL components, forming a chimeric lipoviral particle (LVP). Biochemical and ultrastructural studies demonstrated that infectious HCV particles are coated with endogenous apolipoproteins that play key roles in viral attachment and entry, explaining the higher infectivity of lipoprotein-associated HCV (2).
The heterogeneous size and appearance of extracellular HCV, ranging from 40 to >100 nm in diameter, suggests that the set of associated proteins (both viral and cellular), as well as their stoichiometry, might vary across the virion population. Additionally, the specific infectivity of HCV changes according to the cell system/host that the virus is produced in, highlighting a strong contribution of the host to the makeup of the virus particles (2). Therefore, a proteomic analysis of HCV represents an attractive means of discovering novel virus–host interactions with possible implications for understanding exploitation/subversion strategies that this chronic virus uses to persist within the host.
We recently described two methods for producing and affinity-purifying high titers of cell culture-derived HCV (HCVcc) that enabled ultrastructural analysis of HCV virions (3). The first was based on the construction of an infectious clone with tags fused at the N terminus of E2, whereas the second relied on the use of potent HCV-neutralizing Abs.
In this study, we used both affinity purification approaches to perform proteomic analysis of extracellular HCV virions. We established that Core177 (amino acids 1–177) is the form incorporated in mature HCV particles and detected a number of virion-associated viral and cellular proteins. Functional characterization of HCV-associated cellular proteins identified new host factors, including a nuclear pore complex (NPC) protein, that participate in HCV infection.
Results
Purification of Extracellular HCV Virions.
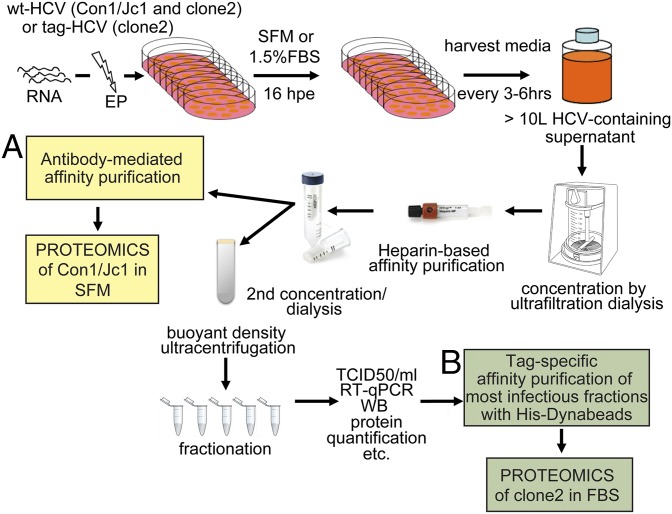
Several challenges hampered compositional studies of patient-derived virus or HCV grown in primary hepatocyte cultures. These challenges include the presence of neutralizing Abs in the plasma of infected patients that interfere with the isolation of circulating virions; the high background from serum proteins; and the limited availability, prohibitive costs, and short survival of primary human hepatocytes in cell culture, as well as their low permissiveness to HCV infection. In contrast, large volumes of HCVcc-containing supernatants can be produced in serum-free or low serum-containing media, and viral envelope proteins can be tagged to facilitate virion affinity purification. Large-scale electroporations of HCV RNA into Huh-7.5.1 hepatoma cells were carried out, and cell supernatants were harvested every 3–6 h up to 120 h postelectroporation and stored at 4 °C to minimize temperature-dependent inactivation of HCV (Fig. 1). Between 12 and 15 L of HCV-containing media was produced and concentrated in a nitrogen-pressurized ultrafiltration cell using low protein binding membranes with a molecular mass cutoff of 100 kDa that retained >85% of the viral infectivity (Fig. S1C and Table S1). Two sequential affinity purification steps were used to enrich for envelope-containing particles. The first one relied on a high-affinity interaction between heparin and E2. Although the heparin-eluted material contained less than 50% of the input viral genomes, a striking increase in the specific infectivity [infectious units (IU) per genome RNAs] of the particles was noted (from ∼1:4,000 for the input material to 1:5 after heparin purification; Fig. S1 B and C and Table S1). The enhanced infectivity suggests that the heparin purification step eliminates noninfectious RNA-containing particles and may additionally remove HCV inhibitors. The heparin column also removed >95% of the protein contaminants present in the concentrated culture supernatant (Fig. S1A). The second affinity purification step consisted of either Ab- or tag-mediated capture of envelope containing HCV particles. In the first case, a Con1/Jc1 virus with five adaptive mutations was produced in serum-free media (SFM), concentrated, heparin-purified, and incubated with a potent HCV-neutralizing Ab that recognizes a discontinuous epitope encompassing E1 and E2 (AR4A) (4) (Fig. 1A). For tag-mediated affinity purification, we used a J6/JFH1 clone 2 infectious genome with a six-histidine (6× His) tag and two copies of a streptavidin tag II fused to the N terminus of E2 (tag-clone 2) (3) that was harvested in 1.5% FBS (Fig. 1B). Tandem affinity purification using His-Dynabeads (Invitrogen) and Streptactin beads (IBA Lifesciences) was attempted but proved unsuccessful. This result might be partly due to particle aggregation postelution (Fig. S1F). His-Dynabeads resulted in lower background than MagStrep beads and were therefore used for tag-mediated virion purification (3). Production of HCV in FBS-containing media yielded higher titer stocks than SFM. Moreover, tag-specific purification methods captured more virions compared with Abs targeting HCV glycoproteins (3) (Fig. 2A). As a result, tag-HCV, but not Con1/Jc1, was subjected to buoyant density fractionation before the second affinity capture step (Fig. 1). Several gradient solutions were tested to achieve best separation of HCV virions from protein contaminants while preserving their infectivity. Iodixanol gradients recovered >90% of the input infectivity in three fractions (Fig. S1E).
Fig. 1.
HCV purification flow chart. Schematic description of the steps used to purify extracellular HCV virions. (A) Con1/Jc1 virus produced in SFM was affinity purified using HCV-neutralizing antibodies prior to proteomics analysis. (B) Tag-clone 2 produced in FBS-containing media was sedimented over a buoyant density gradient and affinity purified with His-Dynabeads. Imidazole-eluted material was subjected to MS. hpi, hours postinfection; qPCR, quantitative PCR; TCID50, 50% tissue culture infectious dose; WB, Western blot.
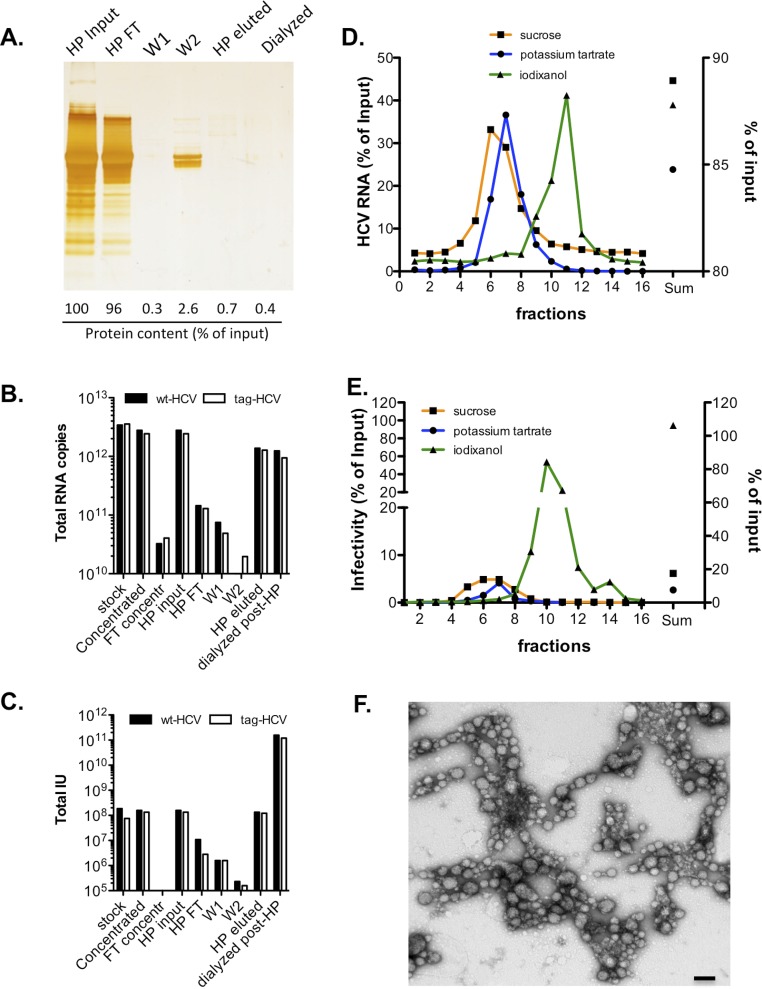
Fig. S1.
Tracking purity and infectivity throughout the purification steps. (A) Proteins in HCV-containing supernatants before and after heparin (HP) purification were separated with 4–12% (wt/vol) SDS-polyacrylamide gel and were visualized by silver staining. Quantification of protein content in each sample was carried out with a Bradford assay and is shown as a percentage of the input sample (stirred cell-concentrated HCV supernatant). FT, flow-through; W, wash. Number of total HCV genomes (B) and total infectious units (IU) (C) recovered during HP purification of wild-type (wt)–HCV and tag-HCV. (D and E) Clone 2 was sedimented through different gradients. The HCV RNA content (D) and infectivity (E) of each fraction were measured and are expressed as a percentage of input sample. The sum of all fractions is shown on the right y axis. (F) Infectious tag-HCV particles from iodixanol gradients were bound to His-Dynabeads, eluted with imidazole, and applied to regular carbon EM grids for negative staining analysis. A representative TEM image shows aggregation of purified HCV particles. (Scale bar, 100 nm.)
Table S1.
Recovery of HCV infectivity and viral RNA throughout the purification protocol
| Sample | Total IU | IU, % | Total RNA | RNA, % | 1/SI |
| Wild-type–HCV | |||||
| Stock | 1.9E + 08 | 100.0 | 3.4E + 12 | 100.0 | 1.8E +04 |
| Concentrated | 1.6E + 08 | 85.3 | 2.8E + 12 | 81.7 | 1.8E +04 |
| FT concentr | 0.0E + 00 | 0.0 | 3.2E + 10 | 1.0 | — |
| HP input | 1.6E + 08 | 100.0 | 2.8E + 12 | 100.0 | 1.8E + 04 |
| HP FT | 1.1E + 07 | 6.8 | 1.4E + 11 | 5.2 | 1.3E + 04 |
| W1 | 1.6E + 06 | 1.0 | 7.5E + 10 | 2.7 | 4.7E + 04 |
| W2 | 2.3E + 05 | 0.1 | 5.9E + 09 | 0.2 | 2.6E + 04 |
| HP eluted | 1.3E + 08 | 84.8 | 1.4E +12 | 49.6 | 1.0E + 04 |
| Dialyzed post-HP | 1.6E + 11 | 99,683.5 | 1.2E + 12 | 44.4 | 7.9E + 00 |
| Tag-HCV | |||||
| Stock | 7.5E + 07 | 100.0 | 3.6E + 12 | 100.0 | 6.6E + 04 |
| Concentrated | 8.4E + 07 | 111.4 | 2.9E + 12 | 82.4 | 3.5E + 04 |
| FT concentr | 0.0E + 00 | 0.0 | 4.0E + 10 | 1.1 | — |
| HP input | 1.3E + 08 | 100.0 | 2.4E + 12 | 100.0 | 1.8E + 04 |
| HP FT | 2.8E + 06 | 2.1 | 1.3E + 11 | 5.3 | 4.6E + 04 |
| W1 | 1.6E + 06 | 1.2 | 4.9E + 10 | 2.0 | 3.1E + 04 |
| W2 | 1.6E + 05 | 0.1 | 2.0E + 10 | 0.8 | 1.2E + 05 |
| HP eluted | 1.2E + 08 | 90.7 | 1.3E + 12 | 52.4 | 1.1E + 04 |
| Dialyzed post-HP | 1.2E + 11 | 89738.8 | 9.5E + 11 | 38.8 | 7.9E + 00 |
FT concentr, flow-through concentration; HP, heparin; 1/SI, 1/specific infectivity; IU, infectious unit(s); W1, wash 1; W2, wash 2.
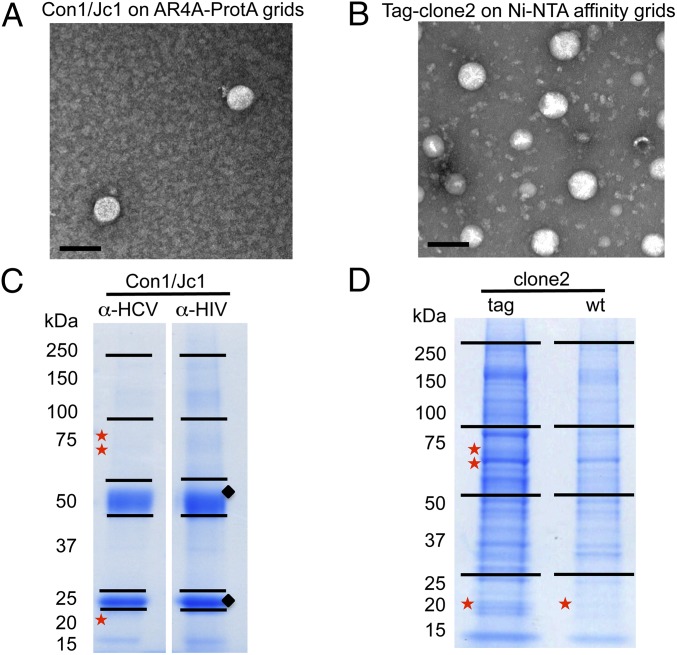
Fig. 2.
Analysis of purified HCV virions. (A and B) Transmission EM images of purified HCV particles used for proteomics studies. (Scale bars, 100 nm.) (C and D) Coomassie staining of SDS/PAGE gels subjected to proteomics analysis. Stars indicate the slices where E2, NS3, and Core (top to bottom) were detected; diamonds show the heavy and light Ab chains. Ni-NTA, nickel-nitrilotriacetic acid; wt, wild type.
Compositional Analysis of HCV Virions by MS.
Transmission EM of negatively stained purified virions, used as input material for proteomics analysis, revealed intact particles (Fig. 2 A and B). Constituent proteins in HCV-enriched samples were resolved by SDS/PAGE and stained with Coomassie (Fig. 2 C and D).
Viral proteins identifed in purified HCV virions.
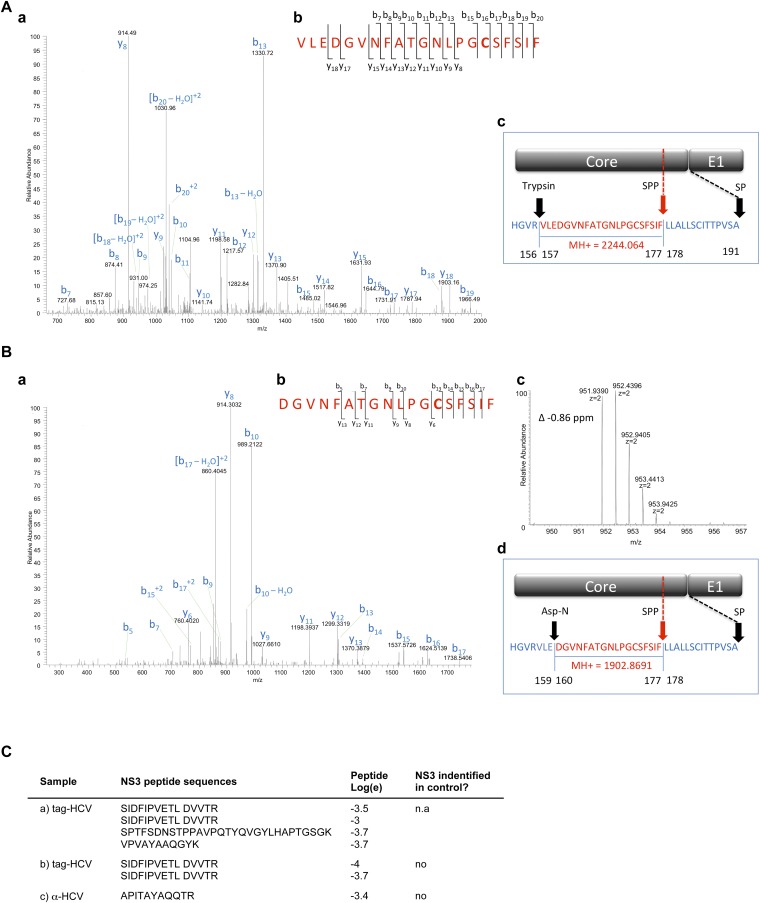
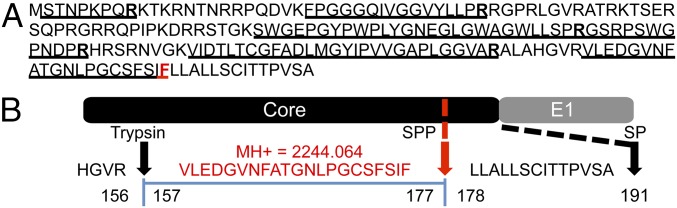
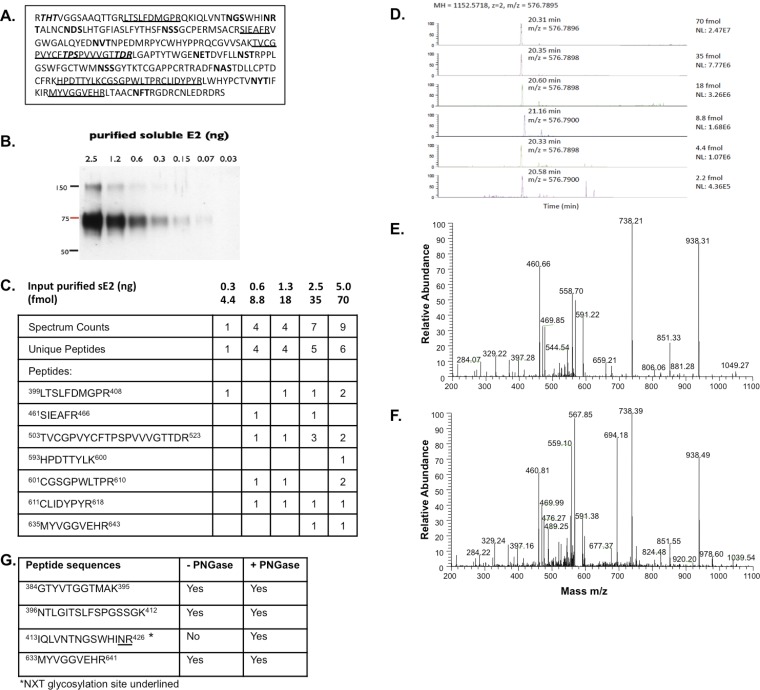
As a first step, selected gel regions where Core, E1, and E2 are expected to migrate (∼21 kDa, 35 kDa, and 70 kDa, respectively) were analyzed by liquid chromatography (LC)/MS to determine whether the purification protocols yielded sufficient material to detect the major HCV structural components. Both purification methods led to the identification of Core, E2, and the viral protease nonstructural 3 (NS3) (Table 1 and Fig. S2C). Table 1 lists the number of observed peptides and the percentage of sequence coverage of the protein (corrected for peptides unlikely to be observed by MS). The statistical score associated with the match, log(e), is also noted. Core displayed the highest relative abundance among the viral proteins using both purification methods, based on the number of unique and total peptides and the sequence coverage achieved. Overall, the sample produced in SFM resulted in a lower LC/MS signal for viral proteins. This result is in agreement with the EM images showing fewer virions in the input material (Fig. 2A). Digestion of virion-associated Core with trypsin resulted in the identification of peptides ending with Arg or Lys, as expected, with the exception of the most C-terminal peptide, which ended with Phe (Fig. 3 and Fig. S2A). To exclude that this cleavage was due to nonspecific trypsin activity, the same sample was digested with Asp-N, with similar results (Fig. S2B). The C terminus of the mature Core protein is produced by the host protease, signal peptide peptidase (SPP), but the form present in mature virions has not been determined (5). Our results indicate that virion-incorporated Core is cleaved at Phe-177. Because E2 is a heavily glycosylated protein, preliminary LC/MS sensitivity tests were conducted using a recombinant soluble E2 ectodomain (6). The identification of unique E2 peptides and total spectra was dose-dependent, with six unique peptides retrieved using the highest amount of purified protein (70 fmol) and a detection limit of ∼4 fmol Fig. S3C. Three of these peptides were also detected from mature virions, using either purification approach (Table 1 and Fig. S3G). In the absence of peptide Peptide-N-Glycosidase F (PNGase F) treatment, no peptides with N-linked glycans were identified, in agreement with previous studies (6). Given the lower amount of input material for the virus preparation in SFM, the gel slices of the Con1/Jc1 sample were cut in two and peptides were extracted with or without in-gel PNGase F digestion. Deglycosylation increased the sensitivity of detection for E2 from three to four unique peptides for the enzyme-treated slice, with the additional peptide containing an NXT glycosylation motif (Fig. S3G). Although no E1 peptides were detected in PNGase F-treated virus preparations, E1 incorporation in virions could be inferred by the fact that the HCV-specific Ab used for the purification recognizes a conformational epitope encompassing E1/E2 (AR4A) (4). Interestingly, the 70-kDa gel region analyzed for E2 detection also contained peptides of the viral protease NS3. Three and one NS3 peptides were retrieved using both tag- and Ab-mediated virus capture, respectively, suggesting that NS3 may be a virion component (Table 1).
Table 1.
Viral proteins in purified HCV virions
| Protein | MS data | Tag-HCV | Wt-HCV | α-HCV | α-HIV |
| E2 | Log (e) | −23 | — | −18 | — |
| Seq cov corr | 34 | — | 35 | — | |
| Unique peps | 3 | — | 3 | — | |
| Spectra | 6 | — | 6 | — | |
| Core | Log (e) | −130 | −9 | −94 | — |
| Seq cov corr | 85 | 14 | 36 | — | |
| Unique peps | 13 | 1 | 9 | — | |
| Spectra | 41 | 2 | 32 | — | |
| NS3 | Log (e) | −3.7 | — | −3.4 | — |
| Unique peps | 1 | — | 1 | — | |
| Spectra | 2 | — | 1 | — | |
| Sequences | SIDFIPVETLDVVTR | APITAYAQQTR | |||
peps, peptides; Seq cov corr, corrected sequence coverage; Wt, wild type.
Fig. S2.
Viral proteins identified in purified HCV virions. (A and B) Identification of the C terminus of mature Core in extracellular HCV virions. LC/MS/MS spectra of peptides at m/z 1,123 (A, a) and m/z 952 (B, a) obtained by digestion of purified tag-HCV particles with trypsin or Asp-N, respectively. X!Tandem searches of the spectra against a database of human and HCV sequences determined that the fragmented peptides correspond to Core residues 157–177 and 160–177, respectively. These peptides are the most C-terminal Core peptides identified in purified HCV samples and the only Core peptides that do not contain the canonical trypsin or Asp-N consensus cleavage site at the carboxyl end. (A, b and B, b) Schematics showing where cleavages in the peptide backbone were observed in the MS/MS spectra. (B, c) MS1 spectrum of the Asp-N peptide that was selected for MS/MS. The error of this precursor mass measurement was −0.86 ppm. (A, c and B, d) Schematic representations of the junction between Core and E1. The observed sequences and calculated protonated masses (MH+) of clone 2 Core peptides generated by cleavage with the exogenous proteases and the host SPP are indicated in red and match the observed data. Full-length Core (191 aa) is generated by cleavage of the signal peptidase (SP), whereas mature Core is produced by SPP-mediated cleavage at position 177. (C) NS3 peptides detected by proteomics analysis of HCV virions after tag- and Ab-mediated affinity purification. n.a., not analyzed.
Fig. 3.
Identification of the C terminus of mature Core in extracellular HCV virions. (A) Amino acid sequence of Core corresponding to residues 1–191 of the J6 HCV polyprotein, genotype 2a. The underlined sequences were identified in the database search. Trypsin cleavage sites are shown in bold, whereas the most C-terminal peptide cleavage site is shown in red. (B) Schematic representation of the junction between Core and E1. The predicted sequence and calculated protonated masses (MH+) of clone 2 Core peptide generated by cleavage with exogenous trypsin protease and the host SPP are indicated in red and match the observed data. Full-length Core (191 aa) is generated by cleavage of the signal peptidase (SP), whereas mature Core is produced by SPP-mediated cleavage at position 177.
Fig. S3.
LC/MS sensitivity test with soluble E2 ectodomain. (A) Amino acid sequence of soluble E2 ectodomain (sE2) corresponding to residues 384–664 of the J6 HCV polyprotein, genotype 2a. The underlined sequences were identified in the database search. The 11 N-linked glycosylation sites are shown in bold, whereas the predicted O-linked glycosylation sites are shown in italics. (B) Western blot analysis of purified sE2. (C) LC/MS analysis of gel slices containing known amounts of sE2. (D) Detection of the E2 peptide 399LTSLFDMGPR408 on an Orbitrap-XL mass spectrometer from gel slices with decreasing femtomoles of sE2. Collision-induced dissociation (CID) fragmentation of the E2 peptide 399LTSLFDMGPR408 from 5 ng of purified sE2 (E) and from purified HCV particles (F) is shown. (G) E2 peptides identified from purified HCV Con1/Jc1 with or without PNGase F treatment.
Cellular proteins identified in purified HCV virions.
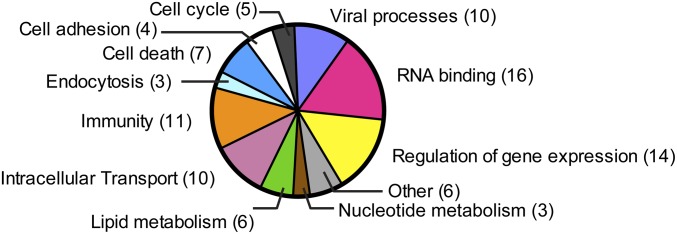
Tag-mediated purification, although successful for isolating intact virions, resulted in high protein background with significant contamination from FBS-derived proteins, hampering the identification of host proteins that specifically associate with HCV. Therefore, we used the HCV-neutralizing Ab AR4A as an alternative approach to purify HCV virions. As a negative control, the same amount of infectious particles was incubated with an HIV-specific Ab (B6). A protein was considered a candidate virion-associated factor if it satisfied all of the following criteria: (i) It is not a known exogenous contaminant, such as keratin; (ii) the log(e) score value does not exceed the log(e) score value of any protein identified from the decoy database of reversed protein sequences; and (iii) both the main protein candidate identified by X!Tandem and its homologs had zero spectrum counts in the corresponding control sample. In total, 46 human proteins were specifically isolated with HCV virions (Table S2). These host factors were grouped into functional categories, including RNA binding, regulation of gene expression, immunological and viral processes, intracellular transport, lipid metabolism, and endocytosis (Fig. 4 and Table S3). Although apolipoproteins (apo) are known components of LVPs, they are not in the list of HCV-associated host factors according to the stringent inclusion criteria, because some peptides were also found in the control lane. Nevertheless, human apoE, apoB-100, apoA-II, apo-CII, and apo-CIII were enriched in purified HCV (Table S4).
Table S2.
Full list of HCV-associated cellular proteins identified by MS
| No. | Identifier | Gene name | Protein name | Log(e) | No. of MS/MS spectra | Molecular mass, kDa | Gel slice |
| 1 | ENSP00000351416 | ANKRD17 | Ankyrin repeat domain 17 | −54.9 | 9 | 274.1 | A |
| 2 | ENSP00000448366 | SCYL2 | SCY1-like 2 | −18.1 | 3 | 77 | A |
| 3 | ENSP00000392423 | RELN | Reelin | −7.9 | 3 | 388.1 | A |
| 4 | ENSP00000451870 | TRAJ56 | T-cell receptor alpha joining 56 | −2.5 | 2 | 2.2 | A |
| 5 | ENSP00000376899 | PTGFRN | Prostaglandin F2 receptor inhibitor | −54.3 | 12 | 98.5 | B |
| 6 | ENSP00000295598 | ATP1A1 | ATPase, Na+/K+ transporting, alpha-1 polypeptide | −27.4 | 5 | 112.8 | B |
| 7 | ENSP00000316032 | NUP98 | Nucleoporin 98kDa | −17.3 | 6 | 195.7 | B |
| 8 | ENSP00000438588 | MTHFD1 | Methylenetetrahydrofolate dehydrogenase (NADP+-dependent) 1 | −13.5 | 4 | 110.5 | B |
| 9 | ENSP00000261973 | RBM25 | RNA binding motif protein 25 | −10.2 | 3 | 100.1 | B |
| 10 | ENSP00000421907 | ADD1 | Adducin 1 (alpha) | −8.2 | 3 | 70 | B |
| 11 | ENSP00000415559 | EGFR | Epidermal growth factor receptor | −6.1 | 3 | 120.6 | B |
| 12 | ENSP00000367122 | SLC3A2 | Solute carrier family 3, member 2 | −23.8 | 4 | 68 | C |
| 13 | ENSP00000264883 | NUP54 | Nucleoporin 54kDa | −14.3 | 6 | 55.4 | C |
| 14 | ENSP00000316664 | IGSF8 | Ig superfamily, member 8 | −8.5 | 2 | 65 | C |
| 15 | ENSP00000441751 | JUP | Junction plakoglobin | −8.3 | 5 | 66.3 | C |
| 16 | ENSP00000388491 | CNOT4 | CCR4-NOT transcription complex, subunit 4 | −8.2 | 2 | 77.8 | C |
| 17 | ENSP00000387282 | AGFG1 | ArfGAP with FG repeats 1 | −7.7 | 3 | 60.7 | C |
| 18 | ENSP00000321347 | STRBP | Spermatid perinuclear RNA binding protein | −4.9 | 2 | 73.6 | C |
| 19 | ENSP00000355890 | EPRS | Glutamyl-prolyl-tRNA synthetase | −4 | 2 | 170.5 | C |
| 20 | ENSP00000368884 | RPS6KA3 | Ribosomal protein S6 kinase alpha-3 | −3.1 | 3 | 83.7 | C |
| 21 | ENSP00000424874 | PGM3 | Phosphoglucomutase-3 | −2.6 | 2 | 59.8 | C |
| 22 | ENSP00000233078 | DAZAP1 | DAZ-associated protein 1 | −8.2 | 2 | 43.4 | D/F |
| 23 | ENSP00000286713 | STOM | Stomatin | −28 | 3 | 31.7 | E |
| 24 | ENSP00000260130 | SDCBP | Syndecan binding protein (syntenin) | −10.7 | 3 | 32.4 | E |
| 25 | ENSP00000221566 | SGTA | Small glutamine-rich tetratricopeptide repeat-containing, alpha | −10.3 | 3 | 34 | E |
| 26 | ENSP00000300161 | YWHAB | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, beta-polypeptide | −6.7 | 2 | 28.1 | E |
| 27 | ENSP00000383981 | RPS27A | Ribosomal protein S27a | −4.4 | 4 | 18 | E |
| 28 | ENSP00000342026 | PRDX6 | Peroxiredoxin 6 | −3.9 | 2 | 25 | E |
| 29 | ENSP00000447938 | CD63 | CD63 molecule | −3.9 | 2 | 16 | E |
| 30 | ENSP00000227525 | TMEM109 | Transmembrane protein 109 | −35.2 | 15 | 26.2 | G |
| 31 | ENSP00000278222 | SAA4 | Serum amyloid A4, constitutive | −12.8 | 3 | 14.7 | G |
| 32 | ENSP00000340761 | BRI3BP | BRI3 binding protein | −11.9 | 3 | 27.8 | G |
| 33 | ENSP00000307705 | HIST1H1E | Histone cluster 1, H1e | −11 | 2 | 21.9 | G |
| 34 | ENSP00000295522 | CLDN1 | Claudin 1 | −10.1 | 3 | 22.7 | G |
| 35 | ENSP00000263645 | CD81 | CD81 molecule | −9.8 | 2 | 25.8 | G |
| 36 | ENSP00000435096 | RPS25 | Ribosomal protein S25 | −9.3 | 3 | 13.7 | G |
| 37 | ENSP00000236671 | CTSD | Cathepsin D | −8.7 | 2 | 44.5 | G |
| 38 | ENSP00000322419 | RPLP2 | Ribosomal protein, large, P2 | −7.6 | 3 | 11.7 | G |
| 39 | ENSP00000400591 | SNRPE | Small nuclear ribonucleoprotein polypeptide E | −7.3 | 2 | 10.8 | G |
| 40 | ENSP00000257013 | FAM127A | Family with sequence similarity 127, member A | −7.2 | 3 | 13.2 | G |
| 41 | ENSP00000360399 | TMEM59 | Transmembrane protein 59 | −6.9 | 2 | 21.8 | G |
| 42 | ENSP00000325376 | HNRNPM | Heterogeneous nuclear ribonucleoprotein M | −6.8 | 2 | 77.5 | G |
| 43 | ENSP00000233596 | REEP6 | Receptor accessory protein 6 | −6.4 | 2 | 20.7 | G |
| 44 | ENSP00000309757 | LPL | Lipoprotein lipase | −5.6 | 2 | 53.1 | G |
| 45 | ENSP00000358089 | TIAL1 | TIA1 cytotoxic granule-associated RNA binding protein-like 1 | −4.8 | 2 | 43.4 | G |
| 46 | ENSP00000261811 | CYSTM1 | Cys-rich transmembrane module containing 1 | −4.8 | 2 | 10.6 | G |
ArfGAP, GTP-ase activating protein for ADP-ribosylation factors; BRI3, brain protein I3; CCR4-NOT, chemokine (C-C motif) receptor 4; DAZ, deleted in azoospermia; FG, phe-gly; TIA1, T-cell restricted intracellular antigen-1. In bold, candidate hits selected for follow-up studies.
Fig. 4.
Functional classification of host proteins in HCV virions according to their known cellular functions.
Table S3.
Functional classification of cellular proteins copurified with HCV virions
| Cellular function | Gene name |
| RNA binding | ADD1, RPLP2, CNOT4, RPS25, DAZAP1, AGFG1, RBM25, SLC3A2, TIAL1, HIST1H1E, SNRPE, RPS27A, EPRS, STRBP, HNRNPM, NUP98 |
| Regulation of gene expression | RELN, RPS27A, EGFR, NUP98, JUP, YWHAB, RPS6KA3, TIAL1, EPRS, TMEM59, DAZAP1, RBM25, RPS25, RPLP2 |
| Immunity | RPS6KA3, RPS27A, EPRS, NUP98, NUP54, YWHAB, EGFR, CTSD, ADD1, SLC3A2, SAA4 |
| Viral processes | NUP54, RPLP2, CLDN1, SGTA, NUP98, ANKRD17, RPS27A, CD81, RPS25, IGSF8 |
| Intracellular transport | NUP54, RPLP2, SCYL2, NUP98, RPS25, AGFG1, SDCBP, YWHAB, CD63, RPS27A |
| Cell death | YWHAB, TMEM109, RPS27A, RPS6KA3, ADD1, RBM25, CTSD |
| Lipid metabolism | LPL, PRDX6, CD81, EGFR, SAA4, PTGFRN |
| Cell cycle | NUP98, NUP54, RPS27A, RPS6KA3, EGFR |
| Cell adhesion | SDCBP, EGFR, JUP, CLND1 |
| Endocytosis | SCYL2, CD63, ADD1 |
| Nucleotide metabolism | MTHFD1, PGM3, ATP1A1 |
| Other | FAM127A, STOM, BRI3BP, SEEP6, CYSTM1, TRAJ56 |
Table S4.
List of apolipoproteins identified by proteomics of HCV virions
| HCV | HIV | ||||||
| Identifier | Protein name | Log(e) | No. of MS/MS spectra | Log(e) | No. of MS/M spectra | Molecular mass, kDa | Gel slice |
| ENSP00000233242 | Human ApoB-100 | −364.2 | 71 | −105.6 | 21 | 515.3 | A |
| ENSP00000252486 | Human ApoE | −120.9 | 50 | −94.1 | 25 | 36.1 | E |
| ENSP00000356969 | Human ApoA-II | −32.5 | 11 | −9.2 | 3 | 11.2 | G |
| ENSP00000227667 | Human Apo-CIII | −31 | 16 | −16.1 | 8 | 10.8 | G |
| ENSP00000252490 | Human Apo-CII | −11.2 | 4 | −3.4 | 2 | 11.3 | G |
| ENSBTAP00000012138 | Bovine ApoA-II precursor | −65.5 | 13 | −18.3 | 4 | 11.2 | G |
Validation of Virion-Associated Host Proteins.
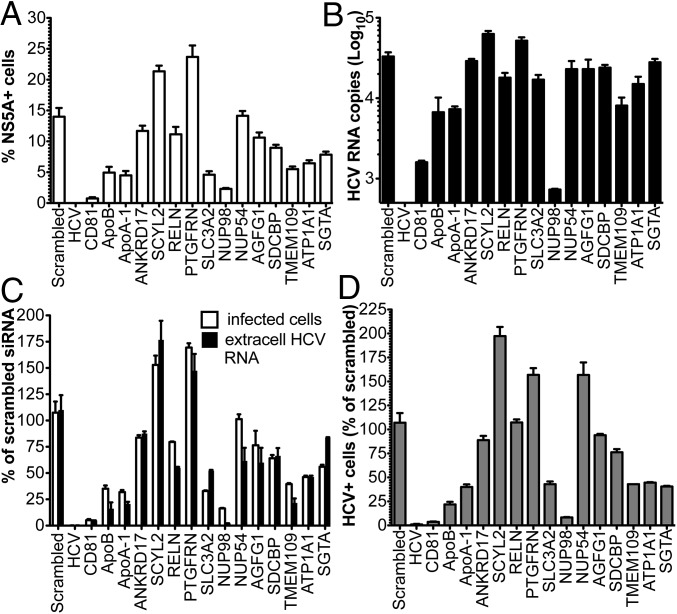
To corroborate the proteomics data, 12 candidate cellular proteins were validated in follow-up studies by RNAi (Table 2). These host factors were prioritized based on the following criteria: (i) observed peptides (n ≥ 3); (ii) log(e) ≤ −7; and (iii) previous implication in HCV or other viral infections, based on PubMed search. The siRNA smart pools targeting the HCV genome and the entry receptor CD81 were used as positive controls for inhibition of viral infection in our functional assays (Fig. 5). ApoB-100 and ApoA-I siRNAs were also included in the analysis, because we previously demonstrated specific incorporation of these host-derived proteins in infectious HCV particles (3). Two days posttransfection with siRNAs, Huh-7.5 cells were challenged with Con1/Jc1 at a low multiplicity of infection (MOI), and the number of NS5A-positive cells and viral genomes released in the culture media was measured 72 h later by fluorescence-activated cell sorting (FACS) and RT-quantitative PCR, respectively (Fig. 5). Therefore, this assay mainly looked at the impact of protein down-regulation on secondary rounds of infection, determining a cumulative effect of the host factors on HCV propagation rather than discriminating between individual steps of the virus life cycle. Cell viability was measured by FACS from the same set of samples at the time of harvesting (Fig. S4B). mRNA expression levels of the targeted host factors were measured by real-time RT-PCR to confirm decreased expression of the intended gene by specific siRNAs (Fig. S4C). A striking decrease in the number of infected cells (Fig. 5 A and C and Fig. S4A) and extracellular viral genomes (Fig. 5 B and C) was observed in cells silenced for the nucleoporin Nup98. This decrease was paralleled by a two-log reduction in viral titers, when cell culture media derived from siRNA-transfected cells was inoculated onto naive Huh-7 cells (Fig. 5D). Interestingly, silencing of SCYL2 and PTGFRN resulted in a 75% and 60% increase in HCV infection, respectively, suggesting an antiviral activity for these host factors. Finally, moderate inhibition of infection (∼50%) was observed in cells where endogenous SLC3A2, TMEM109, ATP1A1, and SGTA were down-regulated.
Table 2.
HCV-associated cellular proteins identified by MS and selected for follow-up studies
| No. | Identifier | Gene name | Protein name | Log (e) | No. of spectra | Molecular mass, kDa | Gel slice |
| 1 | ENSP00000351416 | ANKRD17 | Ankyrin repeat domain 17 | −54.9 | 9 | 274.1 | A |
| 2 | ENSP00000448366 | SCYL2 | SCY1-like 2 | −18.1 | 3 | 77 | A |
| 3 | ENSP00000392423 | RELN | Reelin | −7.9 | 3 | 388.1 | A |
| 4 | ENSP00000376899 | PTGFRN | Prostaglandin F2 receptor inhibitor | −54.3 | 12 | 98.5 | B |
| 5 | ENSP00000295598 | ATP1A1 | ATPase, Na+/K+ transporting, alpha-1 polypeptide | −27.4 | 5 | 112.8 | B |
| 6 | ENSP00000316032 | NUP98 | Nucleoporin 98kDa | −17.3 | 6 | 195.7 | B |
| 7 | ENSP00000367122 | SLC3A2 | Solute carrier family 3, member 2 | −23.8 | 4 | 68 | C |
| 8 | ENSP00000264883 | NUP54 | Nucleoporin 54 kDa | −14.3 | 6 | 55.4 | C |
| 9 | ENSP00000387282 | AGFG1 | ArfGAP with FG repeats 1 | −7.7 | 3 | 60.7 | C |
| 10 | ENSP00000260130 | SDCBP | Syndecan binding protein (syntenin) | −10.7 | 3 | 32.4 | E |
| 11 | ENSP00000221566 | SGTA | Small glutamine-rich tetratricopeptide repeat-containing, alpha | −10.3 | 3 | 34 | E |
| 12 | ENSP00000227525 | TMEM109 | Transmembrane protein 109 | −35.2 | 15 | 26.2 | G |
ArfGAP, GTP-ase activating protein for ADP-ribosylation factors; FG, Phe-Gly.
Fig. 5.
Validation of cellular proteins incorporated in HCV virions by RNAi. Huh-7.5 cells were transfected with siRNA smart pools against the indicated molecules and infected 48 h later with Con1/Jc1 at low MOI (0.05). (A and C) Percentage of HCV+ cells was determined 3 d postinfection (dpi) by NS5A staining and FACS analysis (white bars). (B and C) Number of HCV RNA genomes released in the cell media was quantified by RT-quantitative PCR (black bars). (D) Infectivity of HCV particles produced by siRNA-transfected cells was assessed by inoculating equal volumes of cell culture media on naive Huh-7 cells. The number of NS5A+ cells was determined by FACS 3 dpi. In C and D, data are expressed as a percentage of scrambled siRNA.
Fig. S4.
RNAi of candidate HCV-associated host factors. (A) RNAi in HCV-reporter cells. A Huh-7.5–derived clone encoding the HCV tagRFP-nls-IPS cellular reporter system was transfected with scrambled (a), HCV (b), Nup98 (c), and PTGFRN (d) siRNAs. Silenced cells were infected 2 d later with Con1/Jc1 at low MOI (0.05). Representative images were taken 3 d postinfection (dpi). Uninfected cells display a mitochondrial fluorescent pattern, whereas RFP nuclear translocation upon NS3/4a-mediated cleavage of IPS1 is a signature of productive HCV infection. (Scale bars, 100 μm.) (B) Viability of siRNA-transfected cells at the time of harvesting, as measured by FACS. (C) Percentage of down-regulation of target mRNA levels after siRNA transfection compared with control cells, as measured by RT-quantitative PCR. Values were normalized to GAPDH RNA levels.
Nup98 Interacts with Core and Relocalizes to Cytosolic Lipid Droplets in Infected Cells.
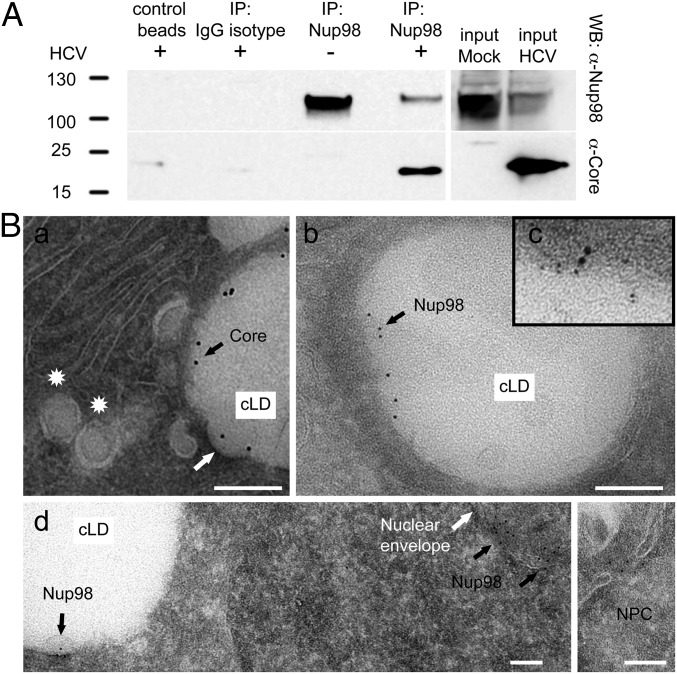
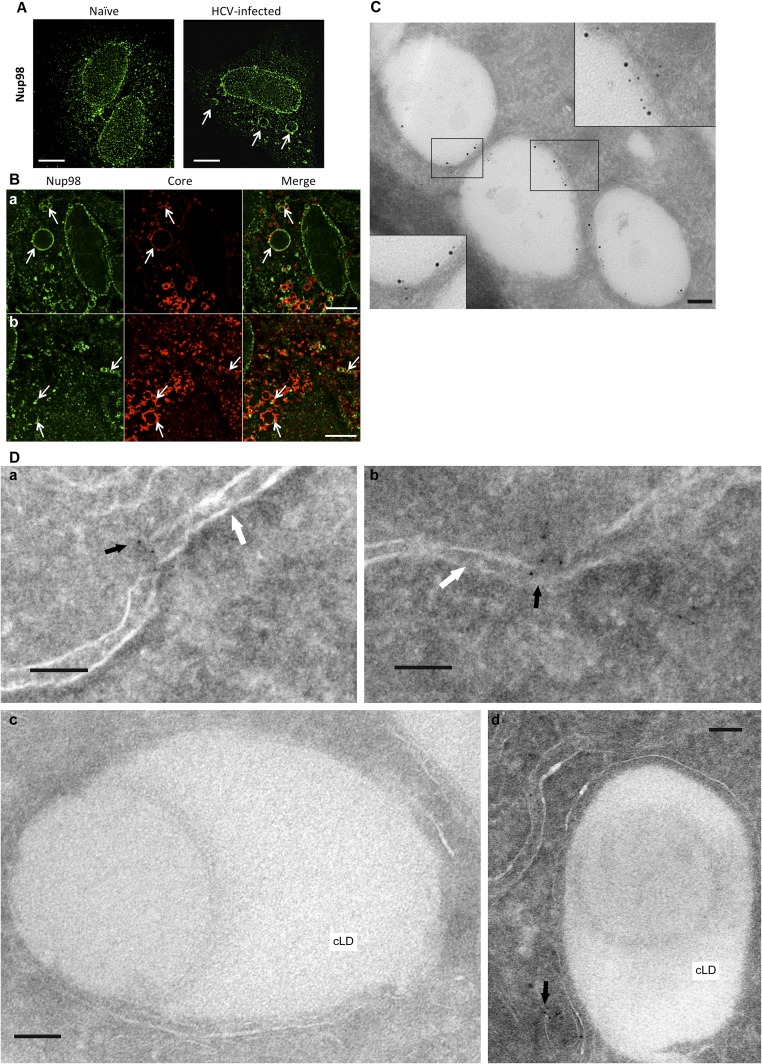
Given the profound inhibition of infection upon Nup98 silencing, we further investigated the role of this protein in HCV biology. Because Nup98 was found in HCV particles, we tested whether it bound to virion structural components. We showed that Core specifically coimmunoprecipitates with endogenous Nup98 (Fig. 6A). In physiological conditions, Nup98 localizes at the nuclear envelope, being a component of the NPC (7). Therefore, the role of Nup98 in HCV infection and its packaging into virions was unexpected considering that HCV replicates in the cytoplasm. To understand where the interaction between Nup98 and Core occurs, we performed immunofluorescence and electron microscopy (iEM) studies of infected Huh-7.5 cells. Differences in Nup98 subcellular distribution were observed at early vs. late stages of infection. Initially, Nup98 staining is comparable to uninfected cells, mainly localizing at the nuclear envelope (Fig. S5 A and D). In contrast, 6 d postinfection, Nup98 is enriched on large, round cytoplasmic structures, where Core also localizes (Fig. S5 A and B). Core is known to accumulate on cytosolic lipid droplets (cLDs), cytoplasmic organelles for lipid storage, before being used for the nucleation of viral capsids (8). To confirm that Core and Nup98 were colocalizing on cLDs, we used the Tokuyasu (9) technique for immunolabeling of cryosections. The expected staining of Core on the surface of cLDs was observed. Furthermore, Nup98 was shown to relocalize to cLDs while retaining its physiological localization at the nuclear envelope (Fig. 6B). Costaining with Core- and Nup98-specific Abs by iEM confirmed that the two proteins colocalize on the surface of cLDs in infected cells (Fig. 6B and Fig. S5C).
Fig. 6.
Nup98 interacts with Core and relocalizes to cLDs in infected cells. (A) Cell lysates from naive and clone 2-infected Huh-7.5 cells (6 dpi) were immunoprecipitated (IP) with anti-Nup98 Ab. Beads without Ab (control beads) or conjugated to isotype-matched IgG served as negative controls. Immunoblot analysis with anti-Nup98 and anti-Core Abs is shown. (B) Huh-7.5 cells infected with clone 2 for 4 d were cryosectioned and stained with Abs to Core (a), Nup98 (b and d), or both (c). Representative iEM images are shown. (a) Core is enriched on cLDs juxtaposed to endoplasmic reticulum (ER) membranes. Membrane curvature may represent a nucleocapsid budding event (white arrow). Spherical particles within vacuoles may be HCV virions at early maturation stage (stars). (c) Costaining with Core-specific (12 nm) and Nup98-specific (6 nm) Abs. (d) Low-magnification image shows the expected localization of Nup98 on both sides of the nuclear envelope, in the central channel of the NPC (Inset), and the additional presence of Nup98 on cLD. (Scale bars, 100 nm.)
Fig. S5.
Subcellular localization of endogenous Nup98 in Huh-7.5 cells. Naive (A) and HCV-infected (B) cells were stained (6 dpi) with anti-Nup98 (green channel) and anti-HCV Core (red channel) Abs. Immunofluorescence images are shown. (Scale bars, 10 μm.) (A) Unusual staining patterns for Nup98 are observed in infected cells (arrows). (B) Colocalization of Nup98 with Core at these circular structures from two independent fields (a and b). (C) Huh-7.5 cells infected with clone 2 for 4 d were cryosectioned and stained with Abs to HCV Core (12 nm) and Nup98 (6 nm), using the Tokuyasu (9) method. Representative iEM images are shown. (Scale bars, 100 nm.) (D) Subcellular localization of endogenous Nup98 in naive Huh-7.5 by iEM. Cells were cryosectioned and stained with anti-Nup98 Ab (6 nm). Nup98 mostly localized at the nuclear membrane (a and b), whereas no staining on the surface of cLDs was observed (c and d). Representative iEM images are shown. Black arrows show Nup98, and white arrows indicate the nuclear membrane. (Scale bars, 100 nm.)
Nup98 Is Required for HCV Morphogenesis.
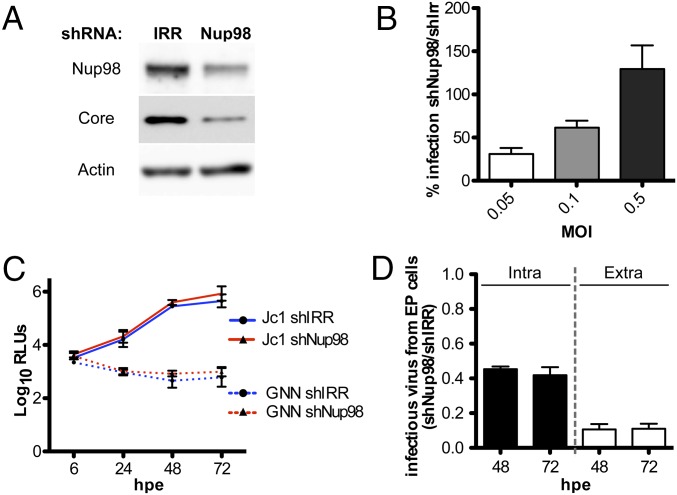
To dissect the role of Nup98 in HCV biology, Huh-7.5 cells were transduced with lentiviral particles encoding either irrelevant shRNA (shIRR) or Nup98-specific shRNA (shNup98) (Fig. 7). The two cell lines were compared using viral assays that looked at specific steps of the HCV life cycle. HCV infection was impaired in cells where Nup98 protein expression was down-regulated, as shown by the reduction of intracellular Core levels (Fig. 7A). The negative impact of Nup98 silencing on infection was overcome in a dose-dependent manner by using higher MOI, suggesting that the block is not at the level of virus entry or early steps of infection (Fig. 7B). Accordingly, using a luciferase-reporter Jc1 virus, we showed that HCV replication and translation were comparable between shIRR and shNup98 cells (Fig. 7C). In contrast, the amount of both intracellular and extracellular infectious particles produced by shNup98 cells was reduced by 50% and 90%, respectively, compared with control cells, indicating a role for Nup98 in the late steps of virion biogenesis (Fig. 7D).
Fig. 7.
Impact of Nup98 on the HCV life cycle. (A) Immunoblot analysis showing Nup98 and Core expression in Huh-7.5 cells expressing either shIRR or shNup98 3 dpi with HCV clone 2. (B) shIRR or shNup98 cells were infected with clone 2 at the indicated MOI for 3 d, and the percentage of NS5A+ cells was determined by FACS analysis. Data are expressed as the percentage of infection measured in shNup98 compared with shIRR. (C and D) ShIRR and shNup98 cells were electroporated with Jc1FLAG2(p7-nsGluc2A) and Jc1FLAG2(p7-nsGluc2A)/GNN RNAs. (C) Kinetics of luciferase secretion in cell culture media were measured between 6 and 72 h postelectroporation (hpe) to monitor viral RNA translation and replication. AR4A was added to the culture media at 1 μg/mL to minimize secondary rounds of infection. (D) Intracellular and extracellular virus harvested from shNup98 and shIRR cells at 48 and 72 hpe was inoculated onto naive Huh-7.5 cells. Infectivity was assessed by luciferase quantification and is shown as the ratio of infection obtained with inocula derived from shNup98 compared with shIRR cells. EP, electroporated.
Discussion
In the past decade, substantial progress in growing HCVcc has been made by using highly permissive cell lines and by selecting more fit viral genomes with adaptive mutations (10). Nevertheless, HCVcc titers remain in the range of 105–106 50% tissue culture infectious dose per milliliter (TCID50/ml), which is ∼100- to 1,000-fold less than other enveloped viruses for which protein composition has been determined (1). If higher virus yields are achieved when serum is added to the culture media, contamination by highly abundant serum proteins compromises the ability to detect cellular proteins that are anticipated to be packaged into HCV virions in low copy numbers (Fig. 2). Furthermore, poor stability of virions makes the purification process extremely time-sensitive, limiting the number of purification steps that can be undertaken before most of the infectivity is lost (11). Finally, propensity to forming aggregates has been observed, preventing tandem affinity purification approaches (Fig. S1).
We undertook appropriate complementary approaches to compositional studies of extracellular HCV virions, using different viral genomes and affinity purification methods. Up to 15 L of virus-containing supernatant was produced for this study, and culture media were harvested frequently and stored at 4 °C to prevent virus inactivation (Fig. 1). More infectious virus particles (approximately 5- to 10-fold) were being produced using FBS-containing media, and these virions were better captured using tag-mediated rather than Ab-mediated purification methods (3). Accordingly, a better sequence coverage of viral proteins was achieved by LC/MS using HCV samples produced in serum. Conversely, HCV isolation via glycoprotein-specific Abs was more effective with preparations in SFM and resulted in significantly cleaner virion pull downs (Fig. 2). However, lower viral titers were achieved in SFM, hampering further fractionation approaches by ultracentrifugation.
Despite these differences, both approaches led to the identification of the same set of viral proteins, with Core displaying the highest sequence coverage (Table 1). Previous studies have shown that Core is cleaved by SPP at Phe-177 and that amino acids 1–177 of the protein are necessary and sufficient for HCV infectivity (5, 12). Using purified HCV virions fractionated over buoyant density gradient, we now determined that the C terminus of Core packaged in infectious particles is Phe-177 (Fig. 3 and Fig. S2).
In addition to the expected structural viral proteins, the protease NS3 was identified in virions produced in FBS as well as SFM, using different purification methods (Table 1 and Fig. S2C). A possible explanation for this finding may be that incoming HCV particles would benefit from having a viral protease that can promptly inactivate cytoplasmic viral sensors upon capsid uncoating, before the formation of the membranous web. Alternatively, given its role in HCV assembly, NS3 may be incorporated in virions by proximity (2). The low signal compared with Core and E2 is consistent with the expectation of the protease not being incorporated in high copy numbers.
The present study reveals an intimate interplay between HCV and the host in the makeup of infectious particles, with 46 potential cellular proteins incorporated into mature virions (Table S2), belonging to a variety of functional classes (Fig. 4 and Table S3). Although it is unclear whether all of these cellular proteins represent true virion components or are only peripherally associated with HCV particles, some of them were previously linked to viral processes (Table S3) and are specifically regulated during HCV infection (13).
Two of these proteins, PTGFRN and SCYL2, display antiviral roles not only against HCV (Fig. 5 and Fig. S4A) but also against other pathogens. PTGFRN (also called CD9P-1 or EWI-F) inhibits the ability of hepatocytes to support Plasmodium sporozoite infection by interacting with CD81 (14). Interestingly, PTGFRN was shown to be up-regulated in HCV-infected cells (13). The IFN-stimulated gene SCYL2 is a component of clathrin-coated vesicles involved in membrane trafficking between the trans-Golgi and endosomes, and it was shown to restrict HIV release by inhibiting the viral protein Vpu (15). Further investigations will be required to determine how these host factors counteract HCV infection and whether they can be considered bona fide restriction factors.
Conversely, Nup98 displayed the strongest proviral phenotype, with a profound reduction in viral genome release and infectious titers upon silencing (Fig. 5). A previous study identified Nup98 as an important host protein for HCV infection (16). Here, we show that Nup98 is copurified with extracellular HCV virions (Table 2), physically interacts with Core (Fig. 6 and Fig. S5), and is specifically required for late stages of the HCV life cycle (i.e., assembly/maturation) (Fig. 7). Follow-up studies will determine whether Nup98’s impact on HCV biogenesis is direct or can also be explained by Nup98 modulation of cellular gene expression.
Nup98 dynamically associates with the NPC, where it regulates nucleocytoplasmic transport of mRNAs and proteins (7). We propose that HCV hijacks Nup98 physiological functions to transport HCV structural components from different cellular compartments, thus coordinating virion morphogenesis. Accordingly, several HCV proteins, including Core, contain functional nuclear localization signal and nuclear export signal sequences that are required to bind cargo proteins and cross the NPC (17). This hypothesis would be in agreement with the model proposed by Neufeldt et al. (16), which suggests the insertion of NPC in the cytoplasm of infected cells to compartmentalize virus replication/assembly. To date, viruses have been described to target the NPC, to cross the nuclear envelope, and/or to control host transcription. In contrast, our proteomics, biochemical, and ultrastructural data suggest a novel mechanism of Nup98 exploitation to aid virus assembly.
In summary, we report the protein composition of extracellular HCV particles. Future studies can address the functional roles that other HCV-associated host factors may fulfill during specific steps of the virus life cycle. These investigations will further our understanding of the host contribution to HCV propagation and pathogenesis.
Materials and Methods
Virion Purification.
HCV stocks were created by electroporating viral RNA into Huh-7.5.1 cells (18). Cells were switched to low-serum media [1.5% (vol/vol) FBS] or SFM 12 h postelectroporation, and virus-containing media were harvested every 3–6 h up to 5 d postelectroporation. Viral supernatants were concentrated in a stirred ultrafiltration cell (Model 8400 with 100-kDa molecular mass cutoff membranes; Millipore) and purified over a heparin column (Hitrap Heparin; GE Healthcare). Heparin-bound HCV was eluted with 0.02 M Tris⋅HCl (pH 7.4) and 0.5 M NaCl, and dialyzed with PBS1X (Invitrogen Gibco) using Amicon Ultra centrifugation devices (regenerated cellulose, low protein binding capacity with 100-kDa molecular mass cutoff; Millipore). Heparin-eluted Con1/Jc1 was incubated with ProtA-coated magnetic beads (Bio-Adembeads; Ademtech), conjugated to Abs specific to HCV (AR4A) or HIV (B6) envelope glycoproteins, and eluted in SDS buffer. Heparin-eluted clone 2-His/one-strep-tag (tag) and wild-type viruses were fractionated over a 10–40% (wt/vol) iodixanol buoyant density gradient (Optiprep; Sigma) (3). Fractions with the highest infectivity were incubated with His-Dynabeads (Invitrogen) for 1 h at room temperature in the presence of 20 mM imidazole and protease inhibitors. Bound particles were washed with 50 mM imidazole and eluted with 900 mM imidazole (in PBS1X, pH 7.7).
Proteomics Analysis.
Gel lanes were sliced in 1-mm intervals, and slices were pooled into six samples. Slices were cubed and destained with 50% (vol/vol) acetonitrile in 50 mM ammonium bicarbonate. To deglycosylate proteins, gel pieces were incubated with 80 μL of PNGase F (11 units/μL; New England BioLabs) in 50 mM sodium phosphate (pH 7.5) at 37 °C overnight and then washed four times with water the next day. Proteins were digested overnight in 3.1 ng/μL trypsin (Promega) in 25 mM ammonium bicarbonate. Peptides were extracted with 2.5 mg/mL POROS R2 20-μm beads (Life Technologies) in 5% (vol/vol) formic acid and 0.2% TFA on a shaker at 4 °C for 24 h. Digests were desalted on Stage Tips (made in-house with 2 layers of octadecyl C18, Empore 2215), eluted, concentrated by SpeedVac (Thermo Savant, SPD111V), and loaded onto a 75-μm ID PicoFrit column (New Objective) packed in-house with 6 cm of reverse-phase C18 material (5-μm particles, 300-Å pores, YMC*Gel ODS-A; YMC). Peptides were gradient-eluted (using 0.1 M acetic acid and 0.1 M acetic acid in 70% (vol/vol) acetonitrile at a flow rate of 200 nL/min) into an LTQ-Orbitrap-XL mass spectrometer (ThermoFisher Scientific) acquiring data-dependent collision-induced dissociation (CID) fragmentation spectra. Using the X!Tandem algorithm, raw data were searched against databases of human, bovine, and HCV protein sequences, as well as a decoy database of reversed protein sequences.
Detailed methods can be found in SI Materials and Methods.
SI Materials and Methods
Cell Cultures.
The human hepatoma cell lines Huh-7, Huh-7.5, and Huh-7.5.1 were cultured as described (18). Huh-7.5/tagRFP-nls (nuclear localization sequence)-IPS (IFN-β promoter stimulator protein 1) cells were generated by transducing Huh-7.5 cells with lentiviral pseudoparticles encoding the tagRFP-nls-IPS reporter that permits visualization of infected cells by nuclear translocation of RFP following IPS cleavage from mitochondria by the HCV NS3/4a protease (19).
Viruses.
HCVcc stocks were produced by electroporation of hepatoma cells and titrated by limiting dilution as described (18). Clone 2 is a cell culture-adapted J6/JFH-1 derivative obtained by serial passages of J6/JFH-1 on Huh-7.5 cells that acquired 12 mutations throughout the polyprotein compared with the parental genome (3). A tagged clone 2 derivative encoding a duplication of amino acids 384 and 385 of the HCV polyprotein; a 6× His repeat; and a one-strep-tag, consisting of two copies of the streptavidin tag II [AGWSHPQFEK(GGGS)2GGGWSHPQFEKGA; Strep II residues in bold] was generated by overlapping PCR and is referred to in this study as tag-HCV. Con1/Jc1 is an intergenotypic chimera between Con1 (genotype 1b) and JFH (genotype 2a) using the Jc1 junction that contains five adaptive mutations: G2833C (NS2, L831F), T2910C (NS2, I857T), A4274G (NS3, I1312V), A6558G (NS5A, K2073R), and A7136C (NS5A, I2266L). Jc1FLAG2(p7-nsGluc2A) is a full-length infectious Jc1 genome with a secreted Gaussia luciferase reporter that can be used to monitor viral translation and replication in transfected or infected cell supernatants (20). Jc1FLAG2(p7-nsGluc2A)/GNN is the corresponding polymerase-defective mutant.
Quantification of HCV Genomes.
Viral RNA was extracted using the QIAamp Viral RNA Kit (Qiagen). HCV genomes were quantified using the EraGen MultiCode-RTx method (EraGen Biosciences) on the LightCycler 480 Real-Time PCR System (Roche Applied Sciences), as previously described (20).
Analysis of HCV Replication, Translation, and Infectivity.
In vitro-transcribed genomic Jc1FLAG2(p7-nsGluc2A) and Jc1FLAG2(p7-nsGluc2A)/GNN RNAs were delivered to Huh-7.5 cells by electroporation using a Bio-Rad Gene Pulser Xcell (Bio-Rad). Luciferase activity in the cell supernatants was quantified using a Renilla Luciferase Assay System (Promega). Electroporated cells were harvested, and intracellular virus was released by four freeze-and-thaw cycles. Intracellular and extracellular virus production was assessed by infecting naive Huh-7.5 cells and measuring luciferase expression 48 h postinfection.
Ultracentrifugation of HCV.
Using a SW41 rotor (Beckman Coulter), 500 μL of a high-titer clone 2 stock was fractionated over: (i) a 10–40% (wt/vol) iodixanol buoyant density gradient (Optiprep; Sigma) for 8 h at 39,000 rpm, (ii) a 10–30% sucrose sedimentation velocity gradient for 1.5 h at 39,000 rpm, and (iii) a 5–25% potassium tartrate sedimentation velocity gradient for 2 h at 39,000 rpm. Sixteen fractions were collected for each gradient, and the number of HCV RNA copies and infectious particles was determined as described above.
Flow Cytometric Analysis.
Cells were harvested using AccuMax (eBioscience), fixed, and permeabilized with Cytofix/CytoPerm buffers (BD Biosciences) according to the manufacturer’s instructions. Cells were incubated for 30 min at room temperature with Alexa Fluor 647 (AF647)- or Alexa Fluor 488 (AF488)-conjugated NS5A Ab (9E10-AF647 and 9E10-AF488, respectively; 1:4,000), washed three times, and resuspended in FACS buffer (PBS, 3% FBS). A BD LSR II flow cytometer and BD FACSDiva software (both from Becton Dickinson) were used for acquisition. Analysis of the data was performed using FlowJo software (FLOWJO, LLC).
RNAi.
ON-TARGETplus smart-pool siRNAs (four per gene; ThermoScientific) targeting HCV-associated host factors were transfected in Huh-7.5 and Huh-7.5/tagRFP-nls-IPS cells using Lipofectamine RNAiMAX (Invitrogen) at a final concentration of 50 nM. Two days posttransfection, cells were infected with Con1/Jc1 (MOI of 0.05). Cells were harvested 3 dpi and stained with 9E10-AF647 for FACS analysis. The viability of siRNA-transfected cells compared with naive cells was measured by FACS at the end point of the experiments (5 d post-siRNA transfection) by acquiring a fixed number of cells for all samples and by calculating the number of events in the live gate population based on the forward scatter (FSC) and side scatter (SSC) parameters.
RT-Quantitative PCR.
RNA from siRNA-transfected cells was extracted at the end point of the experiments (5 d post-siRNA transfection) using an RNeasy kit (Qiagen). Total RNA was reverse-transcribed using a High-Capacity cDNA Reverse Transcription Kit (ThermoFisher). Target gene expression was quantified using TaqMan Gene Expression Assays (ThermoFisher), according to the manufacturer’s instructions. The mRNA levels of the siRNA-targeted host factors were normalized to GAPDH RNA levels to calculate their relative expression compared with control cells.
Generation of Nup98 Down-Regulated Huh-7.5 Cells.
A shRNA duplex sequence targeting the 5′ UTR of the human Nup98 mRNA (5′-CGCGTCCCCGAACCGCGCTTTCTGAAACAATTCAAGAGATTGTTTCAGAAAGCGCGGTTCTTTTTGGAAAT-3′) was cloned into the lentiviral vector pLVTHM (Addgene), downstream of the H1 promoter. The pLVTHM construct encoding the shIRR has been described previously (21). For the production of lentiviral pseudoparticles, HEK-293T cells were cotransfected by calcium phosphate precipitation with the pLVTHM provirus (encoding either shNup98 or shIRR), HIV gag-pol (pCMV-DR8.74), and vesicular stomatitis virus envelope protein G (pMD2G-VSVG) plasmids (Addgene). Huh-7.5 cells were transduced by incubation for 6 h at 37 °C with lentivirus-containing supernatants diluted 1:5 in complete media supplemented with 4 μg/mL polybrene and 20 mM Hepes.
Coimmunoprecipitation and Western Blot.
Huh-7.5 cells were cultured in T75 flasks and infected with HCV clone 2 with an MOI of 10. Six days postinfection, the cells were washed with cold, sterile PBS and then lysed at 4 °C for 1 h in lysis buffer [50 mM Tris HCl (pH 7.5), 50 mM NaCl, 0.5% Triton, 0.2% BSA, Mini EDTA-Free Protease Inhibitor Mixture (Roche)]. Lysates were centrifuged at 20,800 × g at 4 °C for 30 min. Supernatants were incubated overnight at 4 °C with 5 μg of anti-Nup98 (Abcam) and 50 μL of Bio-Adembeads Protein G (Ademtech). Immune complexes were washed three times with washing buffer 1 [50 mM Tris HCl (pH 7.5), 50 mM NaCl, 0.25% Triton, 0.1% BSA] and twice with washing buffer 2 [50 mM Tris HCl (pH 7.5), 50 mM NaCl). Immune complexes and input lysates were boiled for 10 min in loading buffer (Invitrogen), analyzed by SDS/PAGE using 4–12% (wt/vol) Bis/Tris NuPage polyacrylamide gels (Invitrogen), and transferred to nitrocellulose membranes (GE Healthcare). Blots were probed with primary Abs against HCV E2 (1:500; Austral Biologicals), HCV Core (1:1,000; ThermoScientific), and Nup98 (1:1,000; Abcam), followed by HRP-conjugated secondary Abs (1:10,000; JIR), and were developed using Super Signal West Pico Chemiluminescent Substrate (ThermoScientific).
Negative Staining.
To capture wild-type HCV particles, Protein A BioGrids (copper, 400 mesh; Dune Sciences, Inc.) were preincubated with an HCV-specific human IgG (AR4A, 1 μg) according to the manufacturer’s instructions. Fifty microliters of 4% (wt/vol) paraformaldehyde (PFA)-fixed HCV-containing media was incubated on grids for 30 min. To capture tag-HCV particles, affinity grids were prepared as previously described (3). Two percent nickel-nitrilotriacetic acid affinity grids were used for transmission electron microscopy (TEM) studies and floated onto 25–50 μL of PFA-fixed tag-HCV containing 20 mM imidazole for 15–30 min at room temperature. Grids were negatively stained with 2% (wt/vol) uranyl acetate (EMS) and examined in a 100CX JEOL transmission electron microscope (JEOL) with a digital imaging system [XR41-C; Advanced Microscopy Techniques (AMT)] or in a TECNAI Bio-twin spirit G2 (FEI) with a built-in Gatan UltraScan 4000 camera (Gatan, Inc.). Electron micrographs were analyzed with Digital Micrograph (Gatan, Inc.) or AMT software.
Cryosectioning and Immunolabeling.
For TEM immunogold labeling, cell samples were fixed with 4% (wt/vol) PFA and 0.1% (vol/vol) glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 4 h at room temperature and spanned down on 20% (wt/vol) gelatin. Gelatin cell pellets were cryoprotected by incubation in 2.3 M sucrose overnight at 4 °C. Gelatin blocks containing cells were cut further into 1- to 2-mm cubes, mounted on aluminum pins, and cryofixed by plunging into liquid nitrogen. Samples were stored in liquid nitrogen before cryosectioning. Ultrathin sections (70–90 nm thick) were cut using a Leica EM FC6 cryo-ultramicrotome (Leica Microsystems U.K., Ltd.) and mounted on pioloform film-supported nickel grids, according to the Tokuyasu method (9). Sections were immunolabeled using anti-Core (1:5,000) and anti-Nup98 (1:100) primary Abs, followed by a 12-nm colloidal gold anti-mouse Ab or a 6-nm colloidal gold anti-rat secondary Ab, respectively (1:40; Jackson ImmunoResearch). Grids were examined using an FEI Tecnai 12 transmission microscope operated at 120 kV. Images were acquired with an AMT 16000M digital camera.
Acknowledgments
We thank Dr. Joseph Marcotrigiano (Rutgers University) for providing soluble E2, Dr. Mansun Law (The Scripps Research Institute) for the AR4A and B6 Abs, and Harishabd Khalsa for assistance with data analysis. We also thank Dr. Michael Rout (The Rockefeller University) and Dr. Beatriz Fontoura (UT Southwestern) for insightful discussions. This work was supported by NIH grants (to C.M.R.), The Greenberg Medical Research Institute, The Starr Foundation, and Public Health Service Grants GM103314 and GM109824 (to B.T.C.). M.L. was supported by departmental start-up funds (King’s College London). M.T.C. is the recipient of a Rockefeller University Women & Science Fellowship. Funding for M.K. was provided by the Deutsche Forschungsgemeinschaft.
Footnotes
Conflict of interest statement: This paper discusses hepatitis C virus research and tools that were developed in academia and licensed to Apath, LLC, a company in which C.M.R. has equity interest.
This article is a PNAS Direct Submission. R.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518934113/-/DCSupplemental.
References
- 1.Maxwell KL, Frappier L. Viral proteomics. Microbiol Mol Biol Rev. 2007;71(2):398–411. doi: 10.1128/MMBR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindenbach BD, Rice CM. The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol. 2013;11(10):688–700. doi: 10.1038/nrmicro3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catanese MT, et al. Ultrastructural analysis of hepatitis C virus particles. Proc Natl Acad Sci USA. 2013;110(23):9505–9510. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giang E, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci USA. 2012;109(16):6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto K, et al. Intramembrane processing by signal peptide peptidase regulates the membrane localization of hepatitis C virus core protein and viral propagation. J Virol. 2008;82(17):8349–8361. doi: 10.1128/JVI.00306-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whidby J, et al. Blocking hepatitis C virus infection with recombinant form of envelope protein 2 ectodomain. J Virol. 2009;83(21):11078–11089. doi: 10.1128/JVI.00800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffis ER, Xu S, Powers MA. Nup98 localizes to both nuclear and cytoplasmic sides of the nuclear pore and binds to two distinct nucleoporin subcomplexes. Mol Biol Cell. 2003;14(2):600–610. doi: 10.1091/mbc.E02-09-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyanari Y, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9(9):1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 9.Tokuyasu KT. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catanese MT, Dorner M. Advances in experimental systems to study hepatitis C virus in vitro and in vivo. Virology. 2015;479-480:221–233. doi: 10.1016/j.virol.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Ciesek S, et al. How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its susceptibility to chemical biocides. J Infect Dis. 2010;201(12):1859–1866. doi: 10.1086/652803. [DOI] [PubMed] [Google Scholar]
- 12.Kopp M, Murray CL, Jones CT, Rice CM. Genetic analysis of the carboxy-terminal region of the hepatitis C virus core protein. J Virol. 2010;84(4):1666–1673. doi: 10.1128/JVI.02043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond DL, et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6(1):e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charrin S, et al. The Ig domain protein CD9P-1 down-regulates CD81 ability to support Plasmodium yoelii infection. J Biol Chem. 2009;284(46):31572–31578. doi: 10.1074/jbc.M109.057927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyakawa K, et al. Interferon-induced SCYL2 limits release of HIV-1 by triggering PP2A-mediated dephosphorylation of the viral protein Vpu. Sci Signal. 2012;5(245):ra73. doi: 10.1126/scisignal.2003212. [DOI] [PubMed] [Google Scholar]
- 16.Neufeldt CJ, et al. Hepatitis C virus-induced cytoplasmic organelles use the nuclear transport machinery to establish an environment conducive to virus replication. PLoS Pathog. 2013;9(10):e1003744. doi: 10.1371/journal.ppat.1003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin A, et al. Functional characterization of nuclear localization and export signals in hepatitis C virus proteins and their role in the membranous web. PLoS One. 2014;9(12):e114629. doi: 10.1371/journal.pone.0114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindenbach BD, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309(5734):623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 19.Jones CT, et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol. 2010;28(2):167–171. doi: 10.1038/nbt.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrus L, et al. Expression of paramyxovirus V proteins promotes replication and spread of hepatitis C virus in cultures of primary human fetal liver cells. Hepatology. 2011;54(6):1901–1912. doi: 10.1002/hep.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catanese MT, et al. Different requirements for scavenger receptor class B type I in hepatitis C virus cell-free versus cell-to-cell transmission. J Virol. 2013;87(15):8282–8293. doi: 10.1128/JVI.01102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]