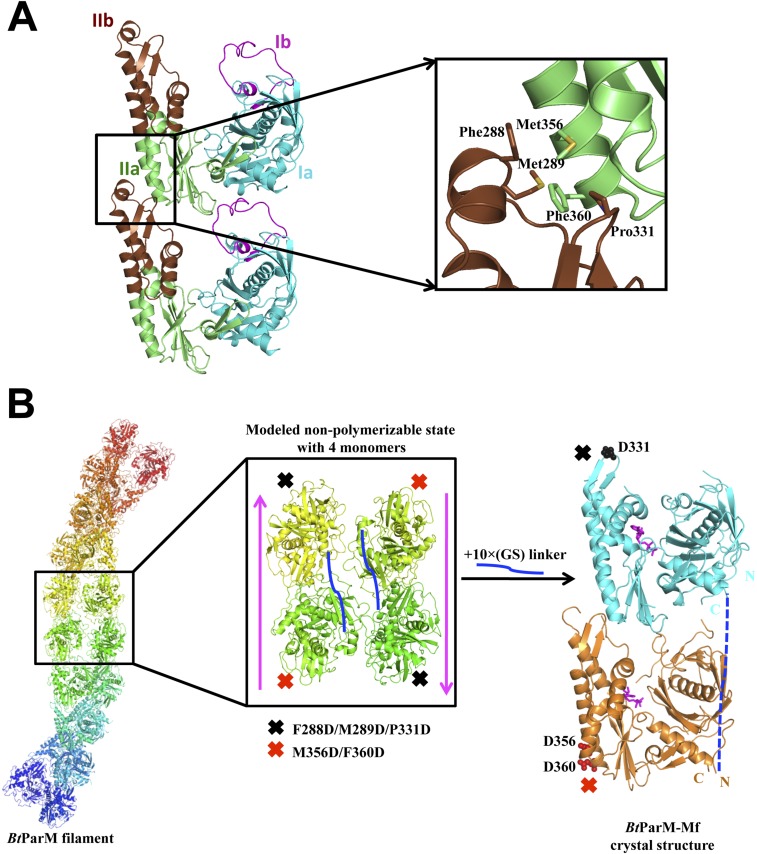
Fig. S8.
Fusion protein strategy with two BtParM mutants to determine the specificity of BtParM:BtParM interactions. (A) Residues involved in the interaction between subdomain IIa of the upper subunit and subdomain IIb of the lower subunit of two protomers from the apo BtParM crystal. Mutations were introduced at the protomer:protomer interface to disrupt crystal contact protofilament formation. BtParM-M1 contains M356D/F360D and BtParM-M2 contains F288D/M289D/P331D. (B) Based on the BtParM filament structures (Left), a fusion strategy was designed to obtain an antiparallel (magenta arrow) nonpolymerizable assembly of four protomers (Center). The two mutants were fused with a 10× (Gly-Ser) linker (solid blue line) in a single construct termed BtParM-Mf. Crystal structure of BtParM-Mf (Right) indicates that the two mutants crystalize as a dimer in the asymmetric unit with similar domain II interactions as in the EM filament structure. Outside this dimer, the protofilament structure was successfully disrupted by the mutations, of which the ordered mutations are depicted. The 10× (Gly-Ser) linker is disordered and indicated by a dashed blue line.