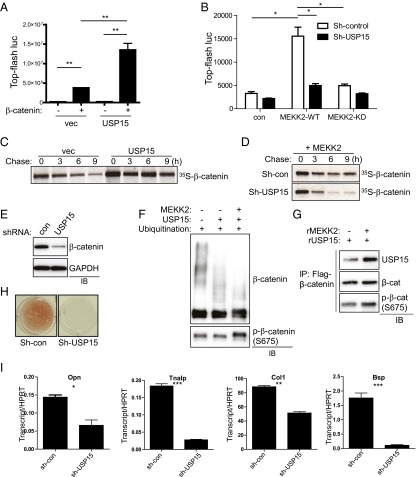
Fig. 4.
USP15 promotes β-catenin stability and osteoblast differentiation. (A) USP15 or a vector control was transfected into HEK293 cells along with TOPflash-luciferase and Renilla in the absence or presence of β-catenin. Luciferase activity was measured 48 h after transfection and normalized to Renilla. P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t test: **P < 0.01. (B) C3H10T1/2 cells were infected with lentivirus expressing control or Usp15 shRNAs, and the resulting cells were transfected with Flag–β-catenin, TOPflash-luciferase, and Renilla in the presence or absence of MEKK2 WT or KD mutant. Luciferase activity was measured 48 h after transfection and normalized to Renilla. P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t tests: *P < 0.05. (C) HEK293 cells were transfected with Flag–β-catenin along with vector or USP15, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography. Numbers indicate the ratio of the densitometric measurement of the corresponding band to the value at time = 0. (D) HEK293 cells were infected with lentiviruses expressing control or Usp15 shRNAs. The resulting cells were transfected with Flag–β-catenin and MEKK2, and β-catenin stability was determined by pulse-chase labeling with [35S]methionine followed by autoradiography. (E) Human MSCs were infected with the indicated lentiviruses and were cultured under osteoblast differentiation conditions for 6 d before immunoblotting. (F) An in vitro ubiquitination assay of β-catenin was performed using recombinant proteins. Activated His–β-catenin was incubated with recombinant SCFSKP1 complex, ubiquitin, ATP, E1, and E2 in the presence or absence of recombinant MEKK2 or USP15. S675 phosphorylation of β-catenin by MEKK2 was confirmed by immunoblotting. (G) An in vitro interaction assay of ubiquitinated β-catenin with USP15 was performed using recombinant proteins. Flag-tagged ubiquitinated β-catenin was incubated with recombinant USP15 in the presence or absence of recombinant MEKK2, immunoprecipitated with anti-Flag antibody–conjugated agarose, and immunoblotted with the indicated antibodies. MEKK2-mediated β-catenin S675 phosphorylation was confirmed by immunoblotting with anti–phospho-β-catenin (S675) antibody. (H and I) Human MSCs were infected with lentiviruses expressing control or Usp15 shRNAs, and the resulting cells were cultured under osteoblast differentiation conditions for 21 d. (H) Alizarin red staining was performed to determine osteoblast mineralization activity. (I) After 10 d of culture, the expression of osteoblast marker genes was analyzed by RT-PCR. For each graph, P < 0.05 by one-way ANOVA. P values for Bonferroni-corrected Student’s t tests: *P < 0.05; **P < 0.01; ***; P < 0.001.