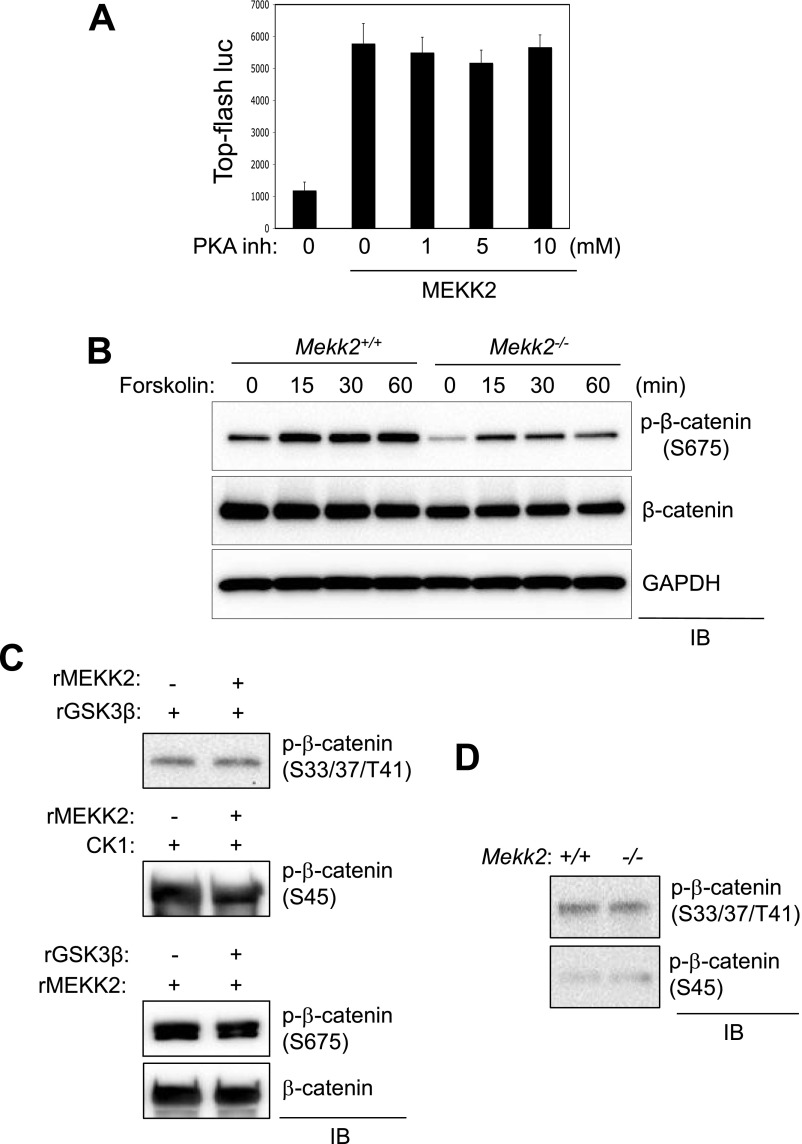
Fig. S5.
MEKK2 functions independently from other known β-catenin kinases in osteoblasts. (A) C3H10T1/2 cells were transfected with a construct encoding MEKK2 along with TOPflash-luciferase and Renilla. Twelve hours after transfection, cells were incubated with different concentrations of the PKA inhibitor H-89, and luciferase activity was measured and normalized to Renilla. (B) Primary COBs isolated from Mekk2+/+ and Mekk2−/− pups were stimulated with 10 μM forskolin at different time points, and lysates were immunoblotted with the indicated antibodies. (C) GST–β-catenin was incubated with recombinant GSK3β (Top) or CK1 (Middle) in the presence or absence of MEKK2, and β-catenin phosphorylation by GSK3β or CK1 was analyzed by immunoblotting with antibodies specific to phospho-β-catenin. (Bottom) GST–β-catenin was incubated with recombinant MEKK2 in the absence or presence of GSK3β, and MEKK2-induced phosphorylation of β-catenin was analyzed by immunoblotting with an anti–phospho-β-catenin (S675) antibody. (D) Primary COBs isolated from Mekk2+/+ and Mekk2−/− pups were lysed and immunoblotted with the indicated antibodies.