Abstract
Background
The transcription factor nuclear factor-κB (NF-κB) is constitutively activated in a variety of human cancers, including gastric cancer. NF-κB inhibitors that selectively kill cancer cells are urgently needed for cancer treatment. Curcumin is a potent inhibitor of NF-κB activation. Unfortunately, the therapeutic potential of curcumin is limited by its relatively low potency and poor cellular bioavailability. In this study, we presented a novel NF-κB inhibitor named Da0324, a synthetic asymmetric mono-carbonyl analog of curcumin. The purpose of this study is to research the expression of NF-κB in gastric cancer and the antitumor activity and mechanism of Da0324 on human gastric cancer cells.
Methods
The expressions between gastric cancer tissues/cells and normal gastric tissues/cells of NF-κB were evaluated by Western blot. The inhibition viability of compounds on human gastric cancer cell lines SGC-7901, BGC-823, MGC-803, and normal gastric mucosa epithelial cell line GES-1 was assessed with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. Absorption spectrum method and high-performance liquid chromatography method detected the stability of the compound in vitro. The compound-induced changes of inducible NF-κB activation in the SGC-7901 and BGC-823 cells were examined by Western blot analysis and immunofluorescence methods. The antitumor activity of compound was performed by clonogenic assay, matrigel invasion assay, flow cytometric analysis, Western blot analysis, and Hoechst 33258 staining assay.
Results
High levels of p65 were found in gastric cancer tissues and cells. Da0324 displayed higher growth inhibition against several types of gastric cancer cell lines and showed relatively low toxicity to GES-1. Moreover, Da0324 was more stable than curcumin in vitro. Western blot analysis and immunofluorescence methods showed that Da0324 blocked NF-κB activation. In addition, Da0324 significantly inhibited tumor proliferation and invasion, arrested the cell cycle, and induced apoptosis in vitro.
Conclusion
The asymmetric mono-carbonyl analog of curcumin Da0324 exhibited significantly improved antigastric cancer activity. Da0324 may be a promising NF-κB inhibitor for the selective targeting of cancer cells. However, further studies are needed in animals to validate these findings for the therapeutic use of Da0324.
Keywords: gastric cancer, anticancer drug, NF-κB inhibitor, asymmetric MACs, apoptosis, curcumin
Introduction
Gastric cancer is the fourth most common cancer and the second most frequent cause of cancer-related mortality worldwide.1 Although surgery is the main treatment regimen for gastric cancer, the effects of operative treatment are unsatisfactory. Postoperative tumor recurrence and lymph node metastasis are often detected after surgery. Chemotherapy is an important treatment option for gastric cancer patients besides surgical resection.2 However, chemotherapy has two main issues that affect the treatment of gastric cancer patients: the resistance of tumor tissue against chemotherapeutic drugs and the toxicity of these drugs to normal tissue.3,4 Thus, gastric cancer therapy should be improved to have a more effective therapeutic schedule.
The nuclear factor (NF)-κB transcriptional pathway is involved in several fundamental biological processes, including immunity,5 inflammation,6 angiogenesis and tumorigenesis,7 and cell proliferation and cell apoptosis.8 In most unstimulated cells, NF-κB is sequestered and inactivated in the cytoplasm when it complexes with one of the specific inhibitors, namely, the IκBs. Many stimuli, such as tumor necrosis factor (TNF)-α9 and lipopolysaccharide,10 as well as some chemotherapeutic drugs,11 can activate NF-κB signaling pathways. This activation is primarily achieved via IκB kinase (IKK)-dependent phosphorylation, which leads to the phosphorylation and degradation of IκBs, and then allows the NF-κB subunit p65 translocate to the nucleus where it exerts its transcriptional activity.12,13 Recent studies have revealed the constitutive activation of NF-κB in several types of cancers, including gastric cancer,14 pancreatic cancer,15 prostate cancer,16 colorectal carcinoma,17 esophageal carcinoma,18 and renal cell carcinoma.19 The activation of NF-κB can lead to downstream protein expression that can promote tumor growth, invasiveness, antiapoptosis, and chemotherapeutic resistance, which eventually allows tumor cells avoid cell death.8,17 Given the antiapoptotic effect of NF-κB, the application of NF-κB inhibitors may prove useful in antitumor therapy. To date, much effort has been focused on developing NF-κB or IKK inhibitors.20,21 For example, the inhibition of constitutive IKK activity by BMS345541 causes the downregulation of NF-κB activity, which in turn leads to the mitochondria-mediated apoptosis of melanoma cells.21
After more than half a century since chemotherapy was introduced for tumor treatment, natural compounds have become an important part of antitumor drugs.22 Curcumin is the active phytochemical derived from the rhizomes of Curcuma longa. Curcumin has attracted much attention because of its surprisingly wide range of beneficial properties, including its antioxidative, anti-inflammatory, and anticancer activity, as well as its pharmacologic safety.23,24 In particular, the effects of curcumin on the NF-κB pathway have been studied in multiple human carcinomas.25,26 Curcumin is a potent inhibitor of NF-κB activation, and inhibition of NF-κB contributes to the cell apoptosis induced by curcumin.25,27,28 Unfortunately, the therapeutic potential of curcumin is limited by its relatively low potency and poor cellular bioavailability.29 Therefore, highly active and clinically promising curcumin analogs should be developed.
In a previous work, our team and several other groups had designed and synthesized a series of curcumin analogs by displacing the β-diketone moiety with a single carbonyl group to produce the symmetric mono-carbonyl analogs of curcumin (MACs; Figure 1A). These symmetric MACs, including B19, B63, EF24, EF31, and F35, demonstrated their effectively increased stability and exhibited better pharmacokinetic activity in vivo.30–34 Furthermore, symmetric MACs, such as EF24, effectively exhibited their antitumor activity by targeting IKK/NF-κB.32 However, subsequent experiments revealed that these symmetric MACs with improved antitumor activity were extremely toxic to normal cells and had poor target selectivity for tumor cells. To obtain lead compounds that specifically targeted cancer cells, we redesigned novel MACs with asymmetric structures (Figure 1A). Fortunately, we discovered these asymmetric MACs retained their high antitumor activity, but showed low toxicity to normal cells. In this study, we report a promising asymmetric MAC named Da0324 that exhibited excellent activity against gastric cancer cells by targeting NF-κB and showed low toxicity to normal epithelial cells in the gastric mucosa.
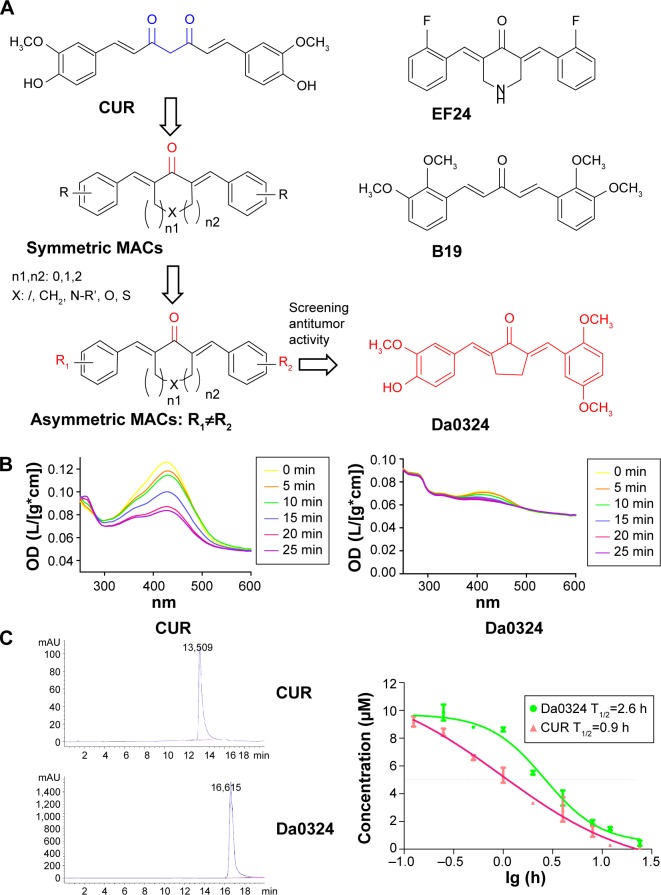
Figure 1.
Da0324 is more stable than curcumin in vitro.
Notes: (A) The chemical structural of curcumin (CUR), asymmetric MACs, and Da0324. (B) Ultraviolet-visible absorption spectra of CUR and Da0324. (C) Time–concentration curves of Da0324 and curcumin detected by the high performance liquid chromatography method in the culture medium.
Abbreviations: h, hours; min, minutes; CUR, curcumin; MACs, mono-carbonyl analogs of curcumin; OD, optical density.
Materials and methods
Chemical synthesis
The chemical synthesis reaction of Da0324 is shown in Figure S1. Purification was achieved by silica gel chromatography to obtain more than 97% purity. The structure of (2E,5E)-2-(2,5-dimethoxybenzylidene)-5-(4-hydroxy-3-methoxybenzylidene)cyclopentanone (Da0324) was characterized by electrospray ionization mass spectrometry and 1H nuclear magnetic resonance (Supplementary material). The chemical structures of curcumin, asymmetric MACs, and Da0324 are shown in Figure 1A. EF24 and B19 were synthesized in our laboratory; their chemical structures are shown in Figure 1A. For the in vitro studies, Da0324 was dissolved in dimethyl sulfoxide (DMSO) solution and prepared at different concentrations.
Stability assay
Da0324 was dissolved and diluted in a DMSO solution and then mixed with the phosphate buffer (pH, 7.4). The optical density values from 250 to 600 nm were determined using a Spectrum Max M5 apparatus. The absorption curves were recorded every 5 minutes for more than 25 minutes. The chemical stability of Da0324 and curcumin in cell culture medium was evaluated by the high performance liquid chromatography method. Briefly, cells were treated with 10 µM Da0324 or 10 µM curcumin in six-well plates. After the indicated time intervals, the culture medium was collected, Da0324 and curcumin were extracted from the culture medium by the addition of ethyl acetate, and their concentrations were determined by the high performance liquid chromatography method using an Agilent LC (1,200 series).
Cell culture, chemical reagents, and antibodies
The SGC-7901, BGC-823, and MGC-803 human gastric cancer cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Wuhan, People’s Republic of China). The normal gastric mucosa epithelial cell line GES-1 was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (GE Healthcare Life Sciences, Logan, UT, USA), 100 U/mL penicillin (Thermo Fisher Scientific), and 100 mg/mL streptomycin (Thermo Fisher Scientific). All cells were cultured and maintained at 37°C under a humidified atmosphere with 5% CO2.
Reagents were obtained from the following sources: TNF-α (Shanghai Universal Biotech Company, Shanghai, People’s Republic of China); DMSO (Sigma-Aldrich Co., St Louis, MO, USA); 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich); BMS345541 (Sigma-Aldrich); crystal violet staining solution (Beyotime, Shangahi, People’s Republic of China); propidium iodide (PI) (Sigma-Aldrich); FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA); NF-κB Activation, Nuclear Translocation Assay Kit (Beyotime); Hoechst 33258 Staining Kit (Beyotime); and basement membrane Matrigel (BD Biosciences).
The primary antibodies used for Western blot analysis were anti-caspase 3, anticleaved-caspase 3, anti-bax, anti-bcl-2, anti-p53, anti-mdm-2, anti-cdc-2, anti-cyclin B1, anti-p-Ikk, anti-Ikk, anti-IκB-α, anti-p65, anti-Lamin B, anti-p-IκB-α, and anti-GAPDH. All antibodies were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) except the anticleaved-caspase 3. The antibody anticleaved-caspase 3 was purchased from Cell Signaling Technology (Danvers, MA, USA). The donkey antigoat IgG-HRP, goat antimouse IgG-HRP, and donkey antirabbit IgG-HRP secondary antibodies were also purchased from Santa Cruz Biotechnology.
Cell viability assay
Cell viability was assessed with the MTT assay following the manufacturer’s protocol. In brief, cells were plated in 96-well plates at ~5,000 cells per well in 100 mL of the corresponding medium. Subsequently, cells were treated with Da0324, curcumin, BMS345541, EF24, or B19 (at 0.096, 0.48, 2.4, 12, and 60 µM, respectively) for 72 hours and then treated with the MTT solution for 4 hours. Finally, the crystals were solubilized with DMSO solution, and the absorbance was recorded at 490 nm using a microplate reader. The cell inhibition ratio (IR) was calculated using the following equation:
where Atreated and Acontrol were the absorbance values of the treated and the DMSO control groups, respectively. half maximal inhibitory concentration (IC50) was defined as the concentration that caused 50% inhibition of cell proliferation; its value was calculated with the GraphPad statistical software.
Hoechst 33258 staining assay
To examine the apoptotic changes in SGC-7901 cells, we performed the Hoechst 33258 staining assay. After treatment with Da0324 or curcumin for 24 hours, the cells were fixed with the stationary buffer for 10 minutes and then washed twice with phosphate-buffered saline (PBS). Cells were subsequently stained with the Hoechst 33258 solution for 15 minutes at 4°C in the dark, washed repeatedly with PBS, and then evaluated under a fluorescence microscope.
Clonogenic assay
The clonogenic assay was used to test each cell in a given population for the ability to undergo unlimited division and form colonies. After treatment with Da0324 or curcumin for 24 hours, cells were transferred to fresh medium and incubation was continued to allow colony formation. After 7 days, the colonies were stained with a crystal violet solution and photographed.
Cell cycle and apoptosis analysis by flow cytometry
The proportions of cells in the G0/G1, S, and G2/M phases were determined by the flow cytometric analysis of DNA content. In brief, after treatment with Da0324 or curcumin for 24 hours, all cells obtained by trypsinization were washed with PBS and suspended in 75% ethanol for 1 hour at −20°C. The obtained cells were washed again, resuspended, and stained with PI for 15 minutes at 4°C in the dark. The cell distribution across the cell cycle was analyzed with a fluorescence-activated cell sorter.
Flow cytometric analysis was also used to quantify the apoptotic cells. In brief, cells were incubated with Da0324 or curcumin for 24 hours. The incubated cells were subsequently collected and washed twice with PBS. The washed cell samples were incubated with Annexin-V for 10 minutes in the dark and then incubated with PI for 5 minutes. Flow cytometric analysis was then performed using a fluorescence-activated cell sorter.
Matrigel invasion assay
The invasiveness of gastric cancer cells was assessed as the invasion of cells through Matrigel-coated transwell inserts. The upper surface of each chamber was coated with the Matrigel basement membrane and air-dried for 2 hours at room temperature. Gastric cancer cells were added to the upper chamber at a cell density of 1×106 cells/mL in RPMI-1640 medium without FBS and treated with Da0324 or curcumin. Meanwhile, RPMI-1640 with 10% FBS was added to the lower chamber. The chambers were incubated for 24 hours at 37°C. At the end of the incubation period, the cells that migrated to the lower surface of the membrane were fixed and stained with crystal violet solution. The cells on the upper surface of the membrane were carefully removed with a cotton swab. Gastric cancer cells that had migrated from the upper chamber to the lower side of the filter were counted under light microscopy. Tumor cell invasiveness was defined as the mean number of cells in three microscopic fields.
Cytoplasmic and nuclear extraction
The cytoplasmic and nuclear extractions were performed according to the reagent manufacturer’s instructions. After 24 hours of incubation in 100 mm plates, cells were treated with Da0324 or BMS345541 for 1 hour before treatment with TNF-α for 1 hour. Cells were then collected with trypsin and washed thrice with ice-cold PBS. The cells were suspended in Buffer A and incubated on ice for 10 minutes before Buffer B was added to the mixture. After additional incubation on ice for 1 minute, the cell samples were centrifuged for 5 minutes at maximum speed (16,000× g). The obtained cytoplasmic extracts (the supernatant) were transferred into fresh tubes. The remnants were resuspended in Buffer C on ice for 40 minutes with occasional vortexing. After the suspension was centrifuged, the supernatant was collected as the nuclear extract and stored at −80°C until further use.
Assessment of NF-κB nuclear translocation
Cells were treated with Da0324 or BMS345541 for 1 hour prior to treatment with TNF-α for 1 hour. The treated cells were then processed for fluorescence microscopy. In brief, cells were washed with ice-cold PBS and fixed in the stationary buffer for 10 minutes at room temperature. Cells were then washed with the washing buffer and blocked using the confining buffer for 1 hour, followed by overnight incubation at 4°C with the rabbit anti-p65 NF-κB antibody. Subsequently, cells were washed again and incubated with antirabbit Cy3 in the dark for 1 hour before staining with DAPI for 5 minutes. After the final wash with the washing buffer, fluorescence microscopy was used to take photomicrographs of the cells. The obtained images were merged, such that the NF-κB subunit p65 was dyed red and the DAPI-stained nuclei appeared blue.
Western blot analysis
Cells were lysed in lysis buffer, and the lysates were clarified by centrifugation (12,000× g) at 4°C for 10 minutes. Equal amounts of the protein extracts were separated by SDS-PAGE. Proteins were detected by overnight incubation in anti-p-Ikk, anti-Ikk, anti-IκB-α, anti-p65, anti-Lamin B, anti-caspase 3, anticleaved-caspase 3, anti-bax, anti-bcl-2, anti-p53, anti-mdm-2, anti-cdc-2, anti-cyclin B1, anti-p-IκB-α, and anti-GAPDH-specific antibodies prior to incubation with the respective secondary antibodies for 1 hour at 37°C. Antibody detection was performed with the ECL-PLUS chemiluminescence detection kit.
Clinical samples
The corresponding tumor and normal tissues of six gastric cancer patients were obtained from The First Affiliated Hospital of Wenzhou Medical University (Wenzhou, People’s Republic of China). The Clinical Research Ethics Committee of The First Affiliated Hospital Of Wenzhou Medical University approved the study. Informed written consent was obtained from all patients. After surgical resection, tissues were immediately frozen fresh and stored in liquid nitrogen. The samples were minced, homogenized, and incubated on ice for 2 hours. Tissues containing equal amounts of proteins were electrophoresed by SDS-PAGE.
Statistical analysis
All data were derived from at least three independent experiments. Student’s t-test was used to assess the differences between sets of data. Probability values below 0.05 were considered significant.
Results
Da0324 is more stable than curcumin in vitro
The decline of absorption spectra at different moments implies the degradation of a compound, which reflects the stability of the said compound to a certain degree. Figure 1B showed that the absorption intensity of the curcumin spectrum significantly decreased in the phosphate buffer, whereas Da0324 was less degraded than curcumin. We then tested the stability of Da0324 in cell culture medium using the high performance liquid chromatography method. Analysis of the concentration–time profiles of curcumin and Da0324 in culture medium revealed a T1/2 of 0.9 and 2.6 hours for curcumin and Da0324, respectively (Figure 1C). It is thus concluded that Da0324 was more stable than curcumin in vitro.
Expression of p65 and IκB-α in gastric cancer tissues and cells
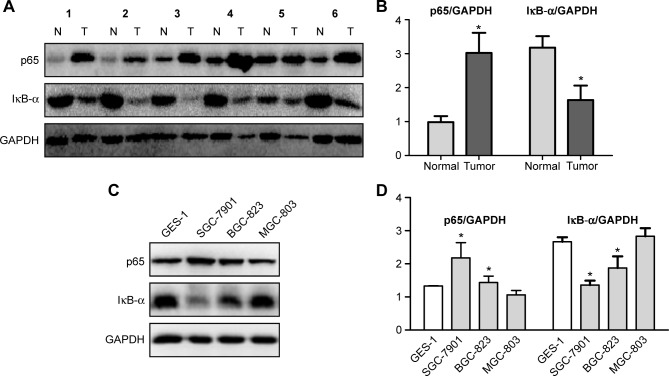
Given that NF-κB is activated in various tumors, the difference between gastric cancer tissues/cells and normal gastric tissues/cells with regard to the state of NF-κB should be evaluated. The expression levels of the NF-κB p65 subunit and IκB-α were investigated in the protein extracts of human gastric cancer tissue samples as compared with the corresponding adjacent normal gastric mucosa. High levels of p65 were found in the tumors, as compared with their corresponding normal tissues. By contrast, the expression of IκB-α was clearly decreased in the cancer tissues compared with the normal tissues (Figure 2A and B), thereby indicating that NF-κB was constitutively activated in human gastric cancer tumors.
Figure 2.
Expression levels of p65 and IκB-α in gastric cancer tissues and cells.
Notes: (A) Expression levels of p65 and IκB-α in human gastric cancer tissue and its corresponding normal adjacent gastric mucosa. (B) Histogram illustrating of the intensity of IκB-α/GAPDG and p65/GAPDH in six human gastric cancer tissues and their corresponding normal adjacent gastric mucosa. *P<0.05 compared with normal tissue. (C, D) The expression levels of p65 and IκB-α in GES-1 normal gastric mucosa epithelial cell and three types of gastric cancer cells. *P<0.05 compared with GES-1.
Abbreviations: N, normal tissue; T, tumor tissue.
The expression levels of p65 and IκB-α in GES-1 normal gastric mucosa epithelial cell and three types of gastric cancer cells (SGC-7901, BGC-823, and MGC-803) were also analyzed with Western blot. The results showed that the expression of p65 increased, whereas that of IκB-α protein decreased in the SGC-7901 and BGC-823 cells, as compared with the GES-1 cells (Figure 2C and D). These results imply the existence of an activated NF-κB signaling pathway in the SGC-7901 and BGC-823 cells.
Da0324 inhibits the cell viability of gastric cancer cells
The inhibition viability of the test compounds on SGC-7901, BGC-823, MGC-803, and GES-1 cells was assessed with the MTT assay. Compared with curcumin, Da0324 displayed higher growth inhibition against several types of gastric cancer cell lines (Table 1). The IC50 values of Da0324 in the SGC-7901, BGC-823, and MGC-803 cells were 3.9±0.9, 4.7±0.6, and 13.3±3.1 µM, respectively, whereas those of curcumin were 22.5±3.9, 17.5±0.5, and 21.2±3.3 µM, respectively. The inhibition ability of Da0324 was better than that of curcumin, particularly in the SGC-7901 and BGC-823 cells. Moreover, the IC50 of Da0324 on GES-1 (27.3±8.2 µM) was close to that of curcumin (29.1±3.8 µM). The toxicity of Da0324 to normal gastric mucosa epithelial cells was visibly similar to that of curcumin, which was previously proven to be nonpoisonous. Therefore, Da0324 may have a selection role to tumor cells. The effects of two symmetric MACs (EF24 and B19) on these cells were also measured. The IC50 values of EF24 on the SGC-7901, BGC-823, MGC-803, and GES-1 cells were 6.1±0.8, 2.3±0.6, 3.1±1.3, and 2.1±0.3 µM, respectively, whereas those of B19 were 2.5±0.6, 3.9±1.3, 4.6±2.2, and 3.7±1.9 µM, respectively. These data showed that symmetric MACs had very good viability and high inhibition activity on gastric cancer cells, but also had high inhibition ability on GES-1 cells. Finally, we tested the viability of the test compounds in terms of its inhibition of BMS345541, an NF-κB inhibitor. The IC50 values of BMS345541 for the SGC-7901, BGC-823, MGC-803, and GES-1 cells were 7.1±1.8, 8.6±1.1, 13.3±6.6, and 9.8±2.5 µM, respectively. Similar to the symmetric MACs, BMS345541 did not have selective effects on tumor and normal cells. However, BMS345541 had a better effect on SGC-7901 and BGC-823 cells, as compared with other gastric cancer cells; similar results were obtained with Da0324. These data encouraged further study on the mechanism of Da0324 activity.
Table 1.
IC50 values of compounds on cells (µM)
| Compound | SGC-7901 | BGC-823 | MGC-803 | GES-1 |
|---|---|---|---|---|
| Da0324 | 3.9±0.9 | 4.7±0.6 | 13.3±3.1 | 27.3±8.2 |
| CUR | 22.5±3.9 | 17.5±0.5 | 21.2±3.3 | 29.1±3.8 |
| EF24 | 6.1±0.8 | 2.3±0.6 | 3.1±1.3 | 2.1±0.3 |
| B19 | 2.5±0.6 | 3.9±1.3 | 4.6±2.2 | 3.7±1.9 |
| BMS345541 | 7.8±1.8 | 8.6±1.1 | 13.3±6.6 | 9.8±2.5 |
Notes: To measure the effects of compounds on the percentage of living cells, gastric cancer cell lines SGC-7901, BGC-823, MGC-803, and normal gastric mucosa epithelial cell line GES-1 were treated with various amounts of Da0324, CUR, BMS345541, EF24, and B19 (0.096, 0.48, 2.4, 12, and 60 µM) for 72 hours, and detected the inhibition rates of tumor cells by MTT. The IC50 values were taken as the concentration that caused 50% inhibition of cell proliferation and calculated by GraphPad statistical software.
Abbreviations: IC50, half maximal inhibitory concentration; CUR, curcumin; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide.
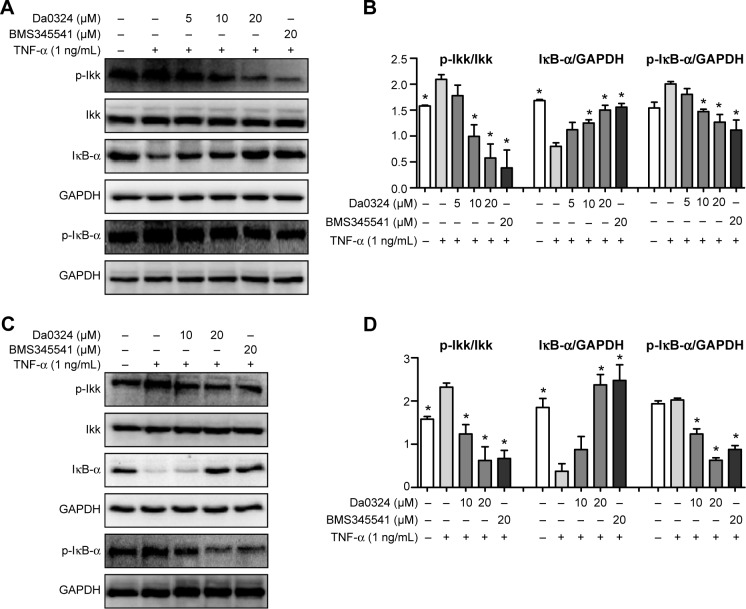
Da0324 inhibits the NF-κB activation induced by TNF-α in gastric cancer cells by suppressing IKK, IκB-α phosphorylation, and inhibiting the degradation of IκB-α in a dose-dependent manner
TNF-α is a cytokine that can promote the classic NF-κB activation.35 BMS345541 is a highly selective IKK inhibitor that can suppress NF-κB activation.21 Consequently, we selected BMS345541 as the positive control. The Da0324-induced downregulation of inducible NF-κB activation in the SGC-7901 and BGC-823 cells was examined by Western blot analysis. Results showed that treatment with Da0324 inhibited the NF-κB activation induced by TNF-α in a dose-dependent manner (Figure 3A–D). A dose of 10 µM Da0324 could significantly inhibit the phosphorylation of IKK and IκB-α; Da0324 also suppressed the degradation of IκB-α that was stimulated by TNF-α in SGC-7901 cells (Figure 3A and B). For the BGC-823 gastric cancer cell line, 10 µM Da0324 could similarly inhibit the expression of p-IKK and p-IκB-α, and inhibit the degradation of IκB-α at concentrations up to 20 µM (Figure 3C and D).
Figure 3.
Da0324 inhibits nuclear factor-κB activation by suppressing IκB kinase (IKK), IκB-α phosphorylation, and inhibiting degradation of IκB-α.
Notes: (A, B) SGC-7901 cells were treated with dimethyl sulfoxide (−, negative control) or BMS345541 (20 µM; +, positive control) or Da0324 (5, 10, or 20 µM) for 60 minutes, then stimulated with tumor necrosis factor-α (TNF-α) (1 ng/mL) for 15 minutes, and then whole-cell extracts were analyzed by Western blot analysis for p-IKK, IκB-α, and p-IκB-α expressions. The intensity of immunoblots digitized by ImageJ software was normalized to IKK and GAPDH, respectively. *P<0.05 compared with TNF-α stimulation group. (C, D) Extracts from BGC-823 cells were analyzed by Western blot analysis. *P<0.05 compared with TNF-α stimulation group.
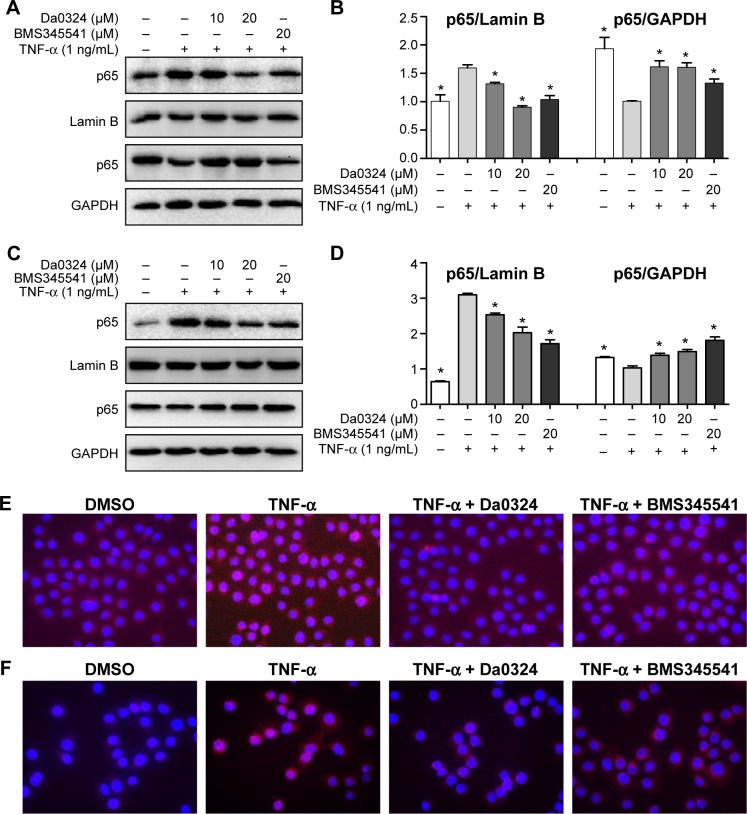
Da0324 inhibits the NF-κB activation induced by TNF-α in gastric cancer cells by inhibiting p65 nuclear translocation in a dose-dependent manner
By measuring the amount of nuclear p65, we can determine whether NF-κB is activated in cells. BMS345541 was also used as a positive control, and Western blot analysis was used to test the inhibition effect of Da0324 on p65 nuclear transfer. Results showed that TNF-α induced the transfer of p65 to the cell nucleus, whereas nuclear p65 did not significantly increase with Da0324 or BMS345541 pretreatment before the TNF-α stimulation in SGC-7901 gastric cancer cells (Figure 4A and B). A similar inhibitory effect was observed in Da0324-treated BGC-823 cells (Figure 4C and D).
Figure 4.
Da0324 inhibits the nuclear factor-κB (NF-κB) activation in gastric cancer cells by inhibiting p65 nuclear translocation.
Notes: (A, B) SGC-7901 cells were treated with dimethyl sulfoxide (DMSO) or BMS345541 or Da0324 (10 or 20 µM) for 60 minutes before stimulated with tumor necrosis factor-α (TNF-α) (1 ng/mL) for 60 minutes, then extracted the proteins of nuclear and cytoplasm, respectively, and the levels of p65 were measured by Western blot analysis. The intensity of nuclear p65 was normalized to Lamin B, and the intensity of cytoplasm p65 was normalized to GAPDH. *P<0.05 compared with TNF-α stimulation group. (C, D) Extracts from BGC-823 cells were analyzed by Western blot analysis. *P<0.05 compared with TNF-α stimulation group. (E) SGC-7901 or (F) BGC-823 cells were treated with DMSO or Da0324 (20 µM) or BMS345541 (20 µM) for 60 minutes, then stimulated with TNF-α (1 ng/mL) for 60 minutes. NF-κB subunit p65 localization was verified by immunofluorescence staining (magnification, 20×10) with p65 antibody.
Subsequently, we used NF-κB nuclear transfer detection kits, such that p65 emitted red fluorescence and the DAPI-stained nuclei emitted blue fluorescence under a fluorescence microscope. The red fluorescence was obviously enhanced in the SGC-7901 cell nuclei with the direct stimulation of TNF-α (Figure 4E). When cells were treated with Da0324 for 1 hour prior to treatment with TNF-α, the red fluorescence did not increase in the cell nuclei. These results also suggest that Da0324 could inhibit the p65 nuclear translocation induced by TNF-α. Similarly, Da0324 and BMS345541 could inhibit the p65 nuclear translocation induced by TNF-α in BGC-823 cells (Figure 4F).
Da0324 inhibits the proliferation of gastric cancer cells
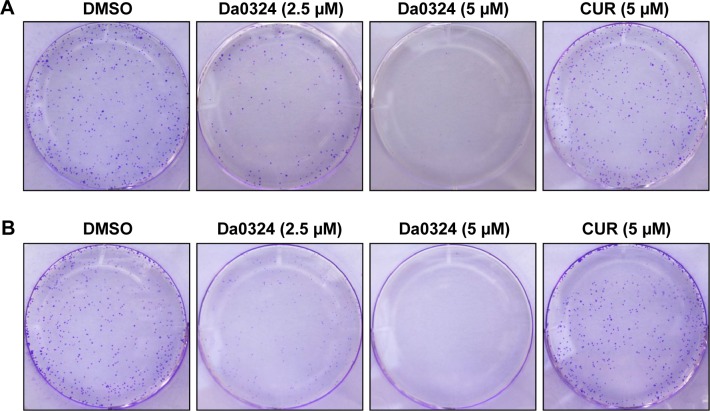
Tumor cells have a capacity for unlimited proliferation, and the effects of compounds on cell proliferation could be studied through clonogenic assays. After treatment with Da0324 or curcumin for 24 hours, cells were transferred to fresh medium and incubation was continued to form single-cell colonies. Results showed that Da0324 inhibited cell colony formation in a dose-dependent manner (Figure 5A and B), and the effect of Da0324 was significantly better than that of curcumin. No colonies were visible after incubation with 5 µM Da0324. By contrast, curcumin at the same concentration had no significant effects on the colonies, as compared with the DMSO group.
Figure 5.
Da0324 inhibits colony formation of gastric cancer cells.
Notes: (A) SGC-7901 or (B) BGC-823 cells were treated with dimethyl sulfoxide (DMSO) or Da0324 (2.5 or 5 µM) or curcumin (CUR) (5 µM) for 24 hours, then replaced with fresh medium, allowed to foster for 1 week, allowed to form colonies, and finally stained by crystal violet and photographs taken.
Da0324 inhibits the invasion of gastric cancer cells
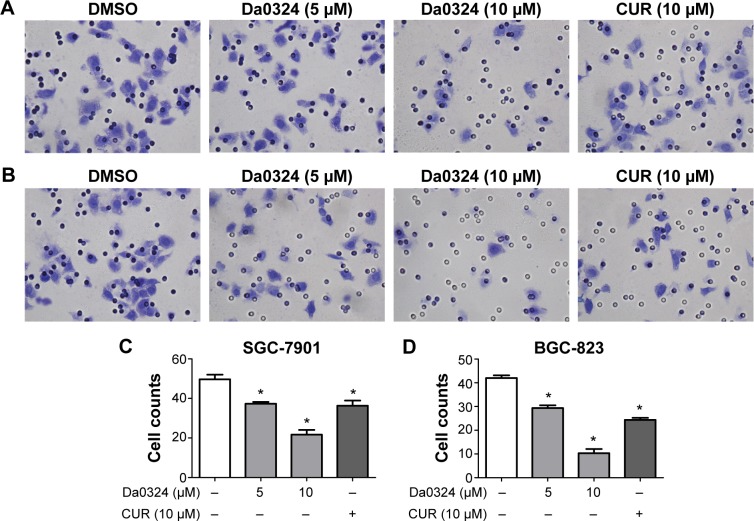
Tumor cells exhibit chemotaxis and will move toward the direction of high nutrient concentrations. We examined whether Da0324 affected the invasive ability of gastric cancer cells using a Matrigel invasion assay. Gastric cancer cells were grown in the upper chamber on an FBS-free medium and treated with Da0324 or curcumin for 24 hours. The number of microscopically visible cells in the lower chamber was significantly reduced in the Da0324-treated group (Figure 6A–D). Da0324 obviously inhibited the invasion of SGC-7901 and BGC-823 cells. Curcumin could similarly inhibit cell invasion, but the effect of Da0324 was stronger than that of curcumin at the same dose.
Figure 6.
Da0324 inhibits invasion of gastric cancer cells.
Notes: (A) SGC-7901 or (B) BGC-823 cells were treated with dimethyl sulfoxide (DMSO) or Da0324 (5 or 10 µM) or curcumin (CUR) (10 µM) for 24 hours, stained by crystal violet and photographs taken under the microscope. (C, D) Histogram illustrating the counts of tumor cells from three separate treatments. *P<0.05 compared with DMSO group.
Da0324 induces cell-cycle arrest in gastric cancer cells
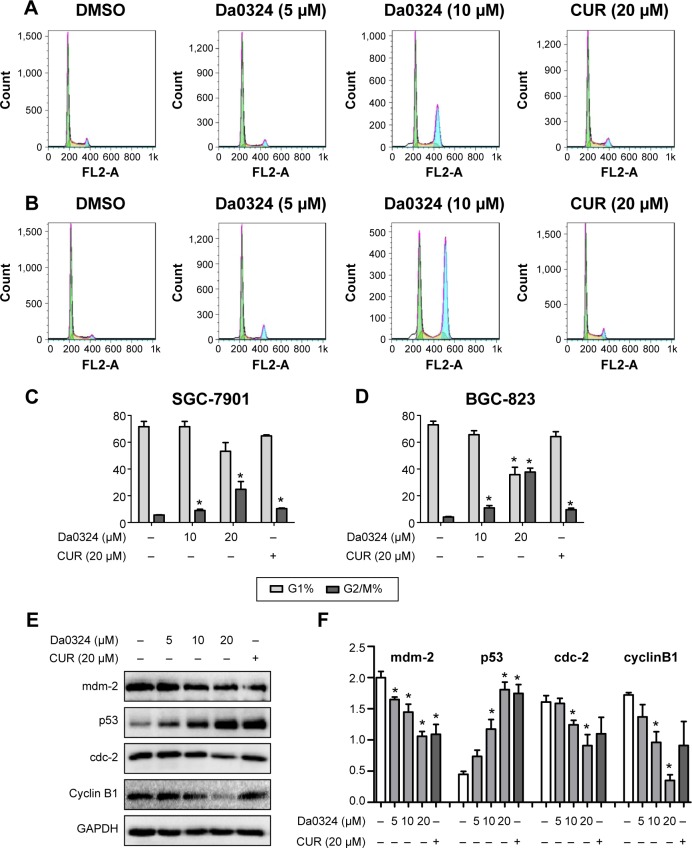
To determine whether Da0324 can alter the cell cycle distribution, we performed flow cytometry. The statistics of the cell numbers in the G0/G1, S, and G2/M phases were analyzed after stimulation with Da0324 or curcumin. Results showed that Da0324 increased the number of cells in the G2/M phase in a dose-dependent manner (Figure 7A–D). Adding 10 µM Da0324 arrested almost 30% of the cell block in the G2/M phase, whereas adding 20 µM curcumin only arrested ~10% of the G2/M block. Therefore, the effect of Da0324 was obviously stronger than that of curcumin.
Figure 7.
Da0324 induces cycle arrest of gastric cancer cells.
Notes: (A) SGC-7901 or (B) BGC-823 cells were treated with dimethyl sulfoxide (DMSO) or Da0324 (5 or 10 µM) or curcumin (CUR) (20 µM) for 24 hours, then all the cells were gathered for staining with propidium iodide. (C, D) Histogram illustrating the rate of G1 and G2/M phases cells from fluorescence-activated cell sorter analysis of three separate treatments. *P<0.05 compared with DMSO group. (E, F) Extracts from SGC-7901 cells treated with DMSO or Da0324 (5, 10, or 20 µM) or CUR (20 µM) for 24 hours were analyzed by Western blot analysis for mdm-2, p53, cdc-2, and cyclin B1 expressions. GAPDH was used for equal protein. *P<0.05 compared with DMSO group.
Furthermore, Western blot analysis of whole-cell extracts from Da0324 or curcumin-treated SGC-7901 cells showed a marked concentration-dependent decrease of the mdm-2, cdc-2, and cyclin B1 proteins after 24 hours of treatment, whereas the p53 protein expression was increased (Figure 7E and F).
Da0324 induces the apoptosis of gastric cancer cells
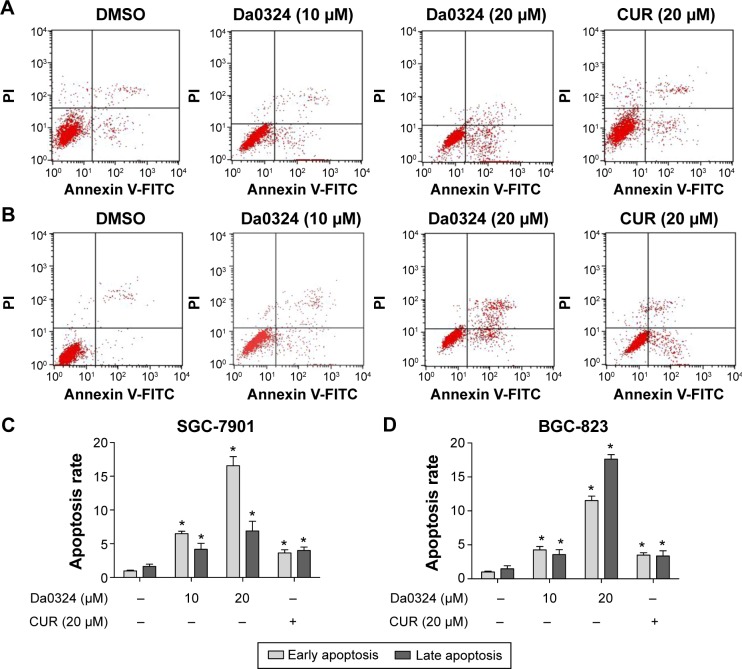
To test the hypothesis that Da0324 can promote cell death by inducing apoptosis, we performed Annexin-V/PI staining and flow cytometric analysis. The number of early- and late-period apoptotic cells was increased in the Da0324-treated group (Figure 8A–D), which was significantly higher than those of the curcumin-treated cells. The Hoechst 33258 staining assay showed that the number of apoptotic nuclei was markedly increased in the SGC-7901 cells of the Da0324-treated group (Figure 8E).
Figure 8.
Da0324 induces apoptosis of gastric cancer cells.
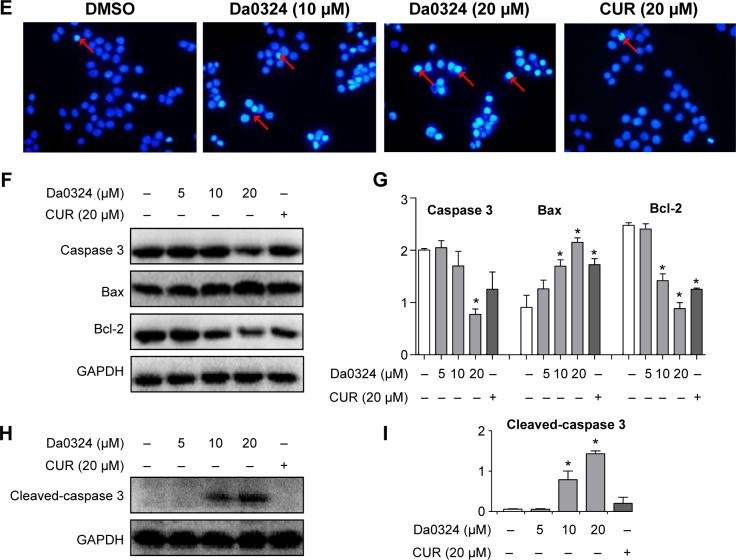
Notes: (A) SGC-7901 or (B) BGC-823 cells were treated with dimethyl sulfoxide (DMSO) or Da0324 (10 or 20 µM) or curcumin (CUR) (20 µM) for 24 hours. Apoptosis was assessed by Annexin V/propidium iodide (PI) staining. Early apoptotic cells were Annexin V-positive (+) and PI-negative (−) cells (lower right section of image), and late apoptosis and death cells were defined as Annexin V-+ and PI-+ cells (upper right section of image). (C, D) Histogram illustrating the rate of apoptosis cells from fluorescence-activated cell sorter analysis of three separate treatments. *P<0.05 compared with DMSO group. (E) SGC-7901 cells were treated with DMSO or Da0324 (10 or 20 µM) or CUR (10 µM) for 24 hours, then stained with Hoechst 33258 staining fluid and took pictures under the microscope. Arrows point to the apoptotic nuclei. (F, G) Extracts from SGC-7901 cells treated with DMSO or Da0324 (5, 10, or 20 µM) or CUR (20 µM) for 24 hours were analyzed by Western blot analysis for proteins caspase 3, bax, and bcl-2 expression. GAPDH was used for equal protein. *P<0.05 compared with DMSO group. (H, I) Extracts from SGC-7901 cells treated with DMSO or Da0324 (5, 10, or 20 µM) or CUR (20 µM) for 24 hours were analyzed by Western blot analysis for protein cleaved-caspase 3. GAPDH was used for equal protein. *P<0.05 compared with DMSO group.
MTT-based antiproliferation assays and flow cytometry all showed that Da0324 induced apoptosis. Thus, we examined the effect of Da0324 on the expression levels of caspase 3, cleaved-caspase 3, and bcl-2, as well as those of the bax proteins, in SGC-7901 cells. Figure 8F–I showed that Da0324 significantly decreased the protein levels of bcl-2 but the bax protein levels increased. At a concentration of 10 µM, Da0324 significantly reduced caspase 3 and induced its cleavage. Our combined results showed that Da0324 potentiated apoptosis in SGC-7901 cells.
Discussion
Although combination therapies are commonly used in the clinic, the outcome of gastric cancer remains poor. On the one hand, only a few gastric cancer patients demonstrate an excellent response to chemotherapeutic drugs, such as cisplatin, primarily because of their resistance to chemotherapy.36 On the other, the adverse effects of chemotherapy often lead to the deterioration of the quality of life in patients. Therefore, a more effective and tolerable therapeutic schedule is needed to improve the clinical outcome. Targeted drugs of low toxicity and high efficiency bring hope to patients; these drugs have become the focus and direction of the most promising treatments for gastric cancer.37 A study on the epidermal growth factor receptor inhibitors cetuximab for the treatment of advanced gastric cancer has entered Phase III in its clinical trials.38 Trastuzumab combined with chemotherapy has become a novel treatment option for Her-2-positive advanced gastric cancer patients.39,40 In addition to the aforementioned drugs, targeted drugs, such as CDKs, mTOR inhibitors, NF-κB inhibitors, C-met inhibitors, IGF-IR inhibitors, and HSP 90 inhibitors, have been the subject of basic or clinical research.41,42
NF-κB is centrally involved in tumorigenesis and tumor progression in various types of cancers.7 Constitutively active NF-κB is associated with tumor growth, metastasis, and chemotherapeutic resistance.17,18 Increasing evidence of the involvement of NF-κB in gastric cancer has accumulated in recent years. The application of NF-κB inhibitor is attracting attention in gastric cancer treatment research.4,43 The nuclear translocation of p65 was significantly high in gastric cancer tissues, as compared with that in the adjacent normal epithelial tissues.14 NF-κB activation was correlated with tumor invasion-related clinicopathological features, such as lymphatic invasion, depth of invasion, peritoneal metastases, and tumor size.14 In the present study, we observed high levels of p65 in gastric cancer tissues and cells, as compared with their normal counterparts (Figure 2). NF-κB was activated by Helicobacter pylori, a well-known risk factor for gastric cancer.44 H. pylori infection promoted gastric cancer cell invasion through the NF-κB- and COX-2-mediated pathways, such that COX-2 or NF-κB inhibitors significantly attenuated the invasiveness of gastric cancer cells as well as the expression of MMP-9 and VEGF proteins.45 The AKT1/NF-κB/Notch1/PTEN axis had an important role in the development of chemoresistance in gastric cancer cells.46 Inhibition of NF-κB activation can directly induce cancer cell apoptosis and reverse drug resistance.47 Chemotherapeutics, such as doxorubicin, activate NF-κB, whereas curcumin potentiated the antitumor effects of doxorubicin in gastric cancer cells by suppressing NF-κB and the NF-κB-regulated antiapoptotic genes bcl-2 and bcl-xL.48 Taken together, these findings implicated the involvement of the NF-κB pathway in gastric cancer. Thus, agents that could modulate NF-κB and the NF-κB-regulated gene products may have an enormous potential for the treatment of gastric cancer. However, the progress of inhibitory drugs that target NF-κB is slow, and thus research and development on NF-κB inhibitors have become urgent.
Recent studies have revealed curcumin is an potent NF-κB inhibitors.28 Curcumin can target NF-κB signaling pathways and downregulate its gene products as well as exert excellent anticancer effects against different types of human tumor cells.27,49 Curcumin also enhanced the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB.50 Combining curcumin with conventional chemotherapeutic agents, such as 5-FU, provided a more effective therapeutic schedule against colon cancer cells; the mechanisms involved were mediated via NF-κB/PI-3K/Src pathways and NF-κB-regulated gene products.50 Moreover, MACs, such as EF24 and AC17, demonstrated inhibitory effects on NF-κB signaling pathways.32,51 A previous study reported that EF24 blocked the nuclear translocation of NF-κB and inhibited TNF-α-induced IκB-α phosphorylation and degradation; furthermore, EF24 directly inhibited the catalytic activity of IKK in an in vitro reconstituted system.32 However, the toxicity on normal cells of symmetric MACs limited their further study. Novel compounds with high efficiency and low toxicity for cancer treatment have become of great interest.
Our current results presented the novel NF-κB inhibitor named Da0324, an asymmetric MAC, which displayed target selectivity for gastric cancer cells. Da0324 significantly inhibited the proliferation of SGC-7901 and BGC-823 gastric cancer cells (Table 1) that had a high level of p65 protein and low expression of IκB-α protein (Figure 2). Compared with curcumin and BMS345541, Da0324 exhibited better activity against SGC-7901 and BGC-823 cells. Da0324 also possessed excellent antitumor activity similar to the active symmetric MACs, EF24, and B19. In addition, Da0324 showed poor inhibition on the normal gastric mucosa epithelial cell line GES-1 which had a low level of p65. However, EF24 and B19 significantly inhibited the proliferation of GES-1 cell, which suggested that symmetric MACs were extremely toxic to normal cells. Da0324 significantly inhibited the TNF-α-induced NF-κB activation by suppressing the phosphorylation of IKK and IκB-α, inhibiting the degradation of IκB-α, and limiting the NF-κB subunit p65 nuclear translocation in gastric cancer cell lines. The inhibitory effect of Da0324 corresponded to that of the IKK-specific inhibitor BMS345541 (Figures 3 and 4). All of these results suggested that the antiproliferative effect of Da0324 may be related to the inhibition of NF-κB signaling pathways, such that Da0324 displayed target selectivity for tumor cells.
The NF-κB pathway regulates several cellular physiological processes, including proliferation, invasion, cell arrest, and apoptosis.8 In this paper, we found that Da0324 can significantly suppress the proliferation, colony formation, and invasion of gastric cancer cells in a dose-dependent manner (Figures 5 and 6) as well as arrest the cell cycle at the G2/M phase (Figure 7) and induce cell apoptosis (Figure 8). All these activities were stronger than curcumin at the same concentration. Inhibitors of the NF-κB pathway can regulate the expression of cell cycle- and apoptosis-related proteins, which cause cell-cycle arrest, growth inhibition, and apoptosis. Therefore, cell cycle- and apoptosis-related proteins were tested. p53 is a pivotal cell fate determinant because of its role in regulating cell cycle progression and apoptosis in response to cellular stress. Mdm-2 is a key negative regulator of p53, which promotes the survival and proliferation of malignant cells via p53 inhibition.52,53 By contrast, the mdm-2 blockade promotes cell-cycle arrest or apoptosis via the enhanced p53 activation and expression of p53 target genes.52 The cyclin B1–cdc-2 complex is a known key regulator of the transition from the G2 to M phase in the cell cycle.54,55 Da0324 decreased the expression of mdm-2 but promoted p53 protein expression in a concentration-dependent manner. The reduced expression of cyclin B1 and cdc-2 proteins after Da0324 treatment was consistent with the observed G2/M arrest (Figure 7). The bcl-2 and caspase protein families are very important for inducing apoptosis.56,57 Da0324 decreased the expression of bcl-2 and promoted bax protein expression. In addition, caspase 3 was activated at a concentration of 10 µM in Da0324-treated SGC-7901 cells. Da0324 was more effective than curcumin at inducing the cleavage of caspase 3 (Figure 8). The decreased levels of the antiapoptotic protein bcl-2, increased amounts of the pro-apoptotic protein bax, and activation of caspase 3 may be involved in the molecular mechanism by which Da0324 induces apoptosis in gastric cells.
Conclusion
An asymmetric MAC that had antigastric cancer activity was described in this study. NF-κB was constitutively activated in gastric cancer tissues and cells. The analog Da0324 partially inhibited the NF-κB activation induced by TNF-α through blocking IKK activity, inhibiting phosphorylation, and degradation of IκB-α in gastric cancer cells. Furthermore, Da0324 limited the accumulation of NF-κB subunit for nuclear translocation. By affecting the NF-κB-regulated downstream products, Da0324 inhibited the proliferation and invasion of gastric cancer cells, caused cycle arrest, and induced apoptosis in vitro. Da0324 exhibited significantly improved antigastric cancer activity over the parent compound curcumin whereas it retained low toxicity. Our data showed that Da0324 is an NF-κB inhibitor that selectively kills gastric cancer cells with activated NF-κB to a certain degree. Altogether, Da0324 is a potential therapeutic agent against cancer. However, further studies are needed in animals to validate these findings for the therapeutic use of Da0324. Although additional investigations are needed to prove the anticancer effects of Da0324, these results provide a new perspective for further investigation of the design and development of asymmetric MAC. Furthermore, our data also offer the potential application of Da0324 and other asymmetric MACs for gastric cancer patient treatment, especially for patients with high NF-κB expression.
Supplementary material
Chemical characterization of Da0324
Yellow powder, 7.4% yield, mp 175.3°C–178.1°C. 1H-NMR (d6-DMSO) δ: 7.954 (s, 1H, β’-H), 7.533 (s, 1H, β-H), 7.260 (s, 1H, H-2), 7.101 (dd, J1 =1.8 Hz, J2 =7.8 Hz, 2H, H-5, H-6), 6.983 (d, J =8.4 Hz, 1H, H-3′), 6.903 (dd, J1 =3.0 Hz, J2 = 9.0 Hz, 1H, H-6′), 6.865 (d, J =9.0 Hz, 1H, H-4′), 3.944 (s, 3H, 3-OCH3), 3.844 (s, 3H, 5′-OCH3), 3.812 (s, 3H, 2′-OCH3), 3.064 (s, 4H, 3″-CH2, 4″-CH2). ESI-MS m/z: 367.0 (M+1)+, calcd for C22H22O5: 366.15.
The chemical synthesis reaction of Da0324.
Abbreviations: ESI-MS, electrospray ionization mass spectrometry; H-NMR, 1hydrogen-nuclear magnetic resonance.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos 81272462, 81202462, and 81302642), the ZheJiang Province Natural Science Fund of China (Grant Nos LY14H160044), and the Technology Foundation for Medical Science of Zhejiang Province (Grant Nos 2012KYA129).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rocco A, Nardone G. Diet, H pylori infection and gastric cancer: evidence and controversies. World J Gastroenterol. 2007;13(21):2901–2912. doi: 10.3748/wjg.v13.i21.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 3.Lai AZ, Cory S, Zhao H, et al. Dynamic reprogramming of signaling upon met inhibition reveals a mechanism of drug resistance in gastric cancer. Sci Signal. 2014;7(322):ra38. doi: 10.1126/scisignal.2004839. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q, Wang J, Zou MJ, et al. Wogonin potentiates the antitumor effects of low dose 5-fluorouracil against gastric cancer through induction of apoptosis by down-regulation of NF-kappaB and regulation of its metabolism. Toxicol Lett. 2010;197(3):201–210. doi: 10.1016/j.toxlet.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 6.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 7.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Sung B. NF-kappaB in cancer: a matter of life and death. Cancer Discov. 2011;1(6):469–471. doi: 10.1158/2159-8290.CD-11-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51(5):1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 10.Choi KC, Lee YH, Jung MG, et al. Gallic acid suppresses lipopolysaccharide-induced nuclear factor-kappaB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol Cancer Res. 2009;7(12):2011–2021. doi: 10.1158/1541-7786.MCR-09-0239. [DOI] [PubMed] [Google Scholar]
- 11.Nakahara C, Nakamura K, Yamanaka N, et al. Cyclosporin-A enhances docetaxel-induced apoptosis through inhibition of nuclear factor-kappaB activation in human gastric carcinoma cells. Clin Cancer Res. 2003;9(14):5409–5416. [PubMed] [Google Scholar]
- 12.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 13.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki N, Morisaki T, Hashizume K, et al. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7(12):4136–4142. [PubMed] [Google Scholar]
- 15.Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer. 2007;97(4):523–530. doi: 10.1038/sj.bjc.6603878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10(24):8460–8464. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto K, Maeda S, Hikiba Y, et al. Constitutive NF-kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res. 2009;15(7):2248–2258. doi: 10.1158/1078-0432.CCR-08-1383. [DOI] [PubMed] [Google Scholar]
- 18.Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24(5):748–754. doi: 10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 19.Sourbier C, Danilin S, Lindner V, et al. Targeting the nuclear factor-kappaB rescue pathway has promising future in human renal cell carcinoma therapy. Cancer Res. 2007;67(24):11668–11676. doi: 10.1158/0008-5472.CAN-07-0632. [DOI] [PubMed] [Google Scholar]
- 20.Yemelyanov A, Gasparian A, Lindholm P, et al. Effects of IKK inhibitor PS1145 on NF-kappaB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene. 2006;25(3):387–398. doi: 10.1038/sj.onc.1209066. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res. 2006;12(3 Pt 1):950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59(6):365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res. 2008;52(Suppl 1):S103–S127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 24.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24(7):1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 26.Sandur SK, Deorukhkar A, Pandey MK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75(2):534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11(20):7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 28.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104(4):879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 29.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Liu Z, Liang G. Promising curcumin-based drug design: mono-carbonyl analogues of curcumin (MACs) Curr Pharm Des. 2013;19(11):2114–2135. [PubMed] [Google Scholar]
- 31.Wang Y, Xiao J, Zhou H, et al. A novel monocarbonyl analogue of curcumin, (1E,4E)-1,5-bis(2,3-dimethoxyphenyl)penta-1,4-dien-3-one, induced cancer cell H460 apoptosis via activation of endoplasmic reticulum stress signaling pathway. J Med Chem. 2011;54(11):3768–3778. doi: 10.1021/jm200017g. [DOI] [PubMed] [Google Scholar]
- 32.Kasinski AL, Du Y, Thomas SL, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74(3):654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Zhang Y, Cai Y, et al. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg Med Chem. 2013;21(11):3058–3065. doi: 10.1016/j.bmc.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Xiao J, Wang Y, Peng J, et al. A synthetic compound, 1,5-bis(2-methoxyphenyl)penta-1,4-dien-3-one (B63), induces apoptosis and activates endoplasmic reticulum stress in non-small cell lung cancer cells. Int J Cancer. 2012;131(6):1455–1465. doi: 10.1002/ijc.27406. [DOI] [PubMed] [Google Scholar]
- 35.Ortis F, Pirot P, Naamane N, et al. Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia. 2008;51(7):1213–1225. doi: 10.1007/s00125-008-0999-7. [DOI] [PubMed] [Google Scholar]
- 36.Cho HJ, Baek KE, Park SM, et al. RhoGDI2 confers gastric cancer cells resistance against cisplatin-induced apoptosis by upregulation of Bcl-2 expression. Cancer Lett. 2011;311(1):48–56. doi: 10.1016/j.canlet.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Onoyama M, Kitadai Y, Tanaka Y, et al. Combining molecular targeted drugs to inhibit both cancer cells and activated stromal cells in gastric cancer. Neoplasia. 2013;15(12):1391–1399. doi: 10.1593/neo.131668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lordick F, Kang YK, Chung HC, Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 39.Kang YK, Rha SY, Tassone P, et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111(4):660–666. doi: 10.1038/bjc.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng L, Tan W, Zhang J, Yuan D, Yang J, Liu H. Combining trastuzumab and cetuximab combats trastuzumab-resistant gastric cancer by effective inhibition of EGFR/ErbB2 heterodimerization and signaling. Cancer Immunol Immunother. 2014;63(6):581–586. doi: 10.1007/s00262-014-1541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang YK, Muro K, Ryu MH, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32(2):355–361. doi: 10.1007/s10637-013-0057-2. [DOI] [PubMed] [Google Scholar]
- 42.Lee KH, Lee JH, Han SW, et al. Antitumor activity of NVP-AUY922, a novel heat shock protein 90 inhibitor, in human gastric cancer cells is mediated through proteasomal degradation of client proteins. Cancer Sci. 2011;102(7):1388–1395. doi: 10.1111/j.1349-7006.2011.01944.x. [DOI] [PubMed] [Google Scholar]
- 43.Shin VY, Jin H, Ng EK, et al. NF-kappaB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis. 2011;32(2):240–245. doi: 10.1093/carcin/bgq240. [DOI] [PubMed] [Google Scholar]
- 44.Maeda S, Yoshida H, Ogura K, et al. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119(1):97–108. doi: 10.1053/gast.2000.8540. [DOI] [PubMed] [Google Scholar]
- 45.Wu CY, Wang CJ, Tseng CC, et al. Helicobacter pylori promote gastric cancer cells invasion through a NF-kappaB and COX-2-mediated pathway. World J Gastroenterol. 2005;11(21):3197–3203. doi: 10.3748/wjg.v11.i21.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou W, Fu XQ, Zhang LL, et al. The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis. 2013;4:e847. doi: 10.1038/cddis.2013.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uetsuka H, Haisa M, Kimura M, et al. Inhibition of inducible NF-kappaB activity reduces chemoresistance to 5-fluorouracil in human stomach cancer cell line. Exp Cell Res. 2003;289(1):27–35. doi: 10.1016/s0014-4827(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 48.Yu LL, Wu JG, Dai N, Yu HG, Si JM. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-kappaB transcription factor. Oncol Rep. 2011;26(5):1197–1203. doi: 10.3892/or.2011.1410. [DOI] [PubMed] [Google Scholar]
- 49.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101(3):1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 50.Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways. PLoS One. 2013;8(2):e57218. doi: 10.1371/journal.pone.0057218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou B, Zuo Y, Li B, et al. Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analog AC17 causes NF-kappaB inhibition and p53 reactivation in human lung cancer cells. Mol Cancer Ther. 2013;12(8):1381–1392. doi: 10.1158/1535-7163.MCT-12-1057. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7(12):979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 53.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 54.Subramaniam D, Natarajan G, Ramalingam S, et al. Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G1025–G1032. doi: 10.1152/ajpgi.00602.2007. [DOI] [PubMed] [Google Scholar]
- 55.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23(16):2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 56.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 57.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351(1–2):41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The chemical synthesis reaction of Da0324.
Abbreviations: ESI-MS, electrospray ionization mass spectrometry; H-NMR, 1hydrogen-nuclear magnetic resonance.