Figure 2.
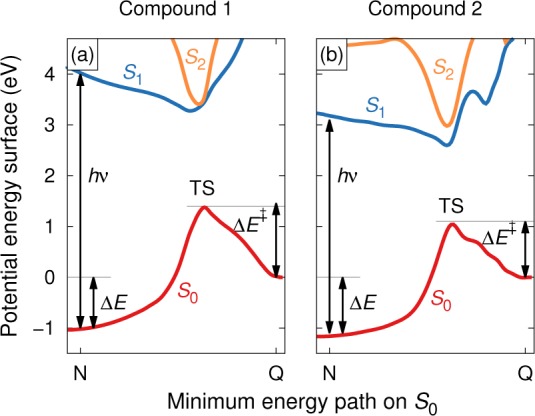
(a) Energy diagram of the parent MOST system (1). The molecule absorbs a photon with minimum energy hν and while in its electronically excited state undergoes an isomerization, forming the photoisomer. If the activation barrier (ΔE⧧) is sufficiently high, the photoisomer is trapped over an extended period of time (i.e., typically months to years). The energy difference between the parent and the photoisomer (ΔE) is the amount of energy stored in the system and is regained as heat when the reversible isomerization is activated, either catalytically or thermally. (b) Energy diagram for a substituted compound (2). Unlike in part a, the transition state geometry was not obtained by full relaxation but constructed following the procedure described in the text. Note that the slices displayed in parts a and b are based on the minimum energy path on S0, whence the excited state surface represents an off-center slice of the conical intersection between S0 and S1. The present visualization is based on (8,8) CASSCF nudged elastic band calculations of the singlet ground state (S0) surface followed by 4-state averaged multistate CASPT2 calculations.
