Abstract
Conventional therapy of primary bone tumors includes surgical excision with wide resection, which leads to physical and aesthetic defects. For reconstruction of bone and joints, allografts can be supplemented with mesenchymal stem cells (MSCs). Similarly, adipose tissue transfer (ATT) is supplemented with adipose-derived stem cells (ADSCs) to improve the efficient grafting in the correction of soft tissue defects. MSC-like cells may also be used in tumor-targeted cell therapy. However, MSC may have adverse effects on sarcoma development. In the present study, human ADSCs, MSCs and pre-osteoclasts were co-injected with human MNNG-HOS osteosarcoma cells in immunodeficient mice. ADSCs and MSCs, but not the osteoclast precursors, accelerated the local proliferation of MNNG-HOS osteosarcoma cells. However, the osteolysis and the metastasis process were not exacerbated by ADSCs, MSCs, or pre-osteoclasts. In vitro proliferation of MNNG-HOS and Saos-2 osteosarcoma cells was increased up to 2-fold in the presence of ADSC-conditioned medium. In contrast, ADSC-conditioned medium did not change the dormant, quiescent state of osteosarcoma cells cultured in oncospheres. Due to the enhancing effect of ADSCs/MSCs on in vivo/in vitro proliferation of osteosarcoma cells, MSCs may not be good candidates for osteosarcoma-targeted cell therapy. Although conditioned medium of ADSCs accelerated the cell cycle of proliferating osteosarcoma cells, it did not change the quiescent state of dormant osteosarcoma cells, indicating that ADSC-secreted factors may not be involved in the risk of local recurrence.
Keywords: Mesenchymal stem cell, Osteosarcoma, Adipose tissue transfer, Cell cycle, Quiescence
Graphical abstract
1. Introduction
High-grade osteosarcoma is an aggressive primary malignant bone tumor that is associated with a relatively good outcome; since pre-operative and adjuvant combination chemotherapy has been introduced the survival rate at 5 years for the non-metastatic form at diagnosis has been 50–70% [1], [2]. Osteosarcoma treatment comprises surgical excision with wide resection of the tumor after neo adjuvant chemotherapy. Adjuvant chemotherapy is then adapted to histological response [3]. Concerning surgical techniques, limb sparing is currently the preferred option. Reconstruction is dependent on resection site: if epiphysis cannot be preserved, mega prosthesis sometimes associated with an allograft is typically used [4]. Otherwise, different conservative techniques are described: massive autograft [5], vascularized autograft sometimes associated with allograft or isolated allograft [6], [7], [8].
A permanent remission from osteosarcoma can be anticipated after 10 years of event free survival [9], [10], [11], [12], [13], where after the primary challenge is to ameliorate the quality of life of patients suffering from physical and aesthetic defects caused by tumor resection. For recovery of damaged soft tissues, plastic and reconstructive surgery includes autologous grafts of adipose tissue. Regenerative medicine promises new alternatives through the use of mesenchymal stem cells (MSCs), which are bone marrow-resident and multipotent cells. They have been originally identified as a source of bone progenitor cells, but they also differentiate into adipocytes, chondrocytes and myoblasts. Human MSCs may be combined with scaffolds to increase bone healing as reported previously [10], [12]. Moreover, the use of MSCs cultured from bone marrow to supplement an osteoarticular allograft in patients treated after bone tumor resection did not increase the risk of local tumor recurrence compared to control populations. Additionally, MSCs can be modified to express tumor-targeted agents [14], [15] and used as “mesenkillers” [16].
Adipose tissue represents an alternative source of MSC-like cells, avoiding the problems of pain, morbidity and low cell number associated with bone marrow harvest [17]. In plastic and reconstructive surgery, adipose tissue-derived stromal cells (ADSCs) may be used to increase in situ survival of the autologous adipose-tissue graft [18], [19], [20], [21], [22], [23]. ADSC have also been utilized as cellular delivery vehicles in bone reconstruction [24].
The use of adjuvant MSC-like cells in the treatment of osteosarcoma may be an important therapeutic issue for patients with lung metastasis associated with poor outcome (30% survival rate at 5 years) [25]. However, the impact of unmodified MSCs on tumor progression remains unpredictable [26]. For instance, it has been observed that rat and human MSCs can promote tumor growth and metastasis in osteosarcoma models [27], [28], [29], [30].
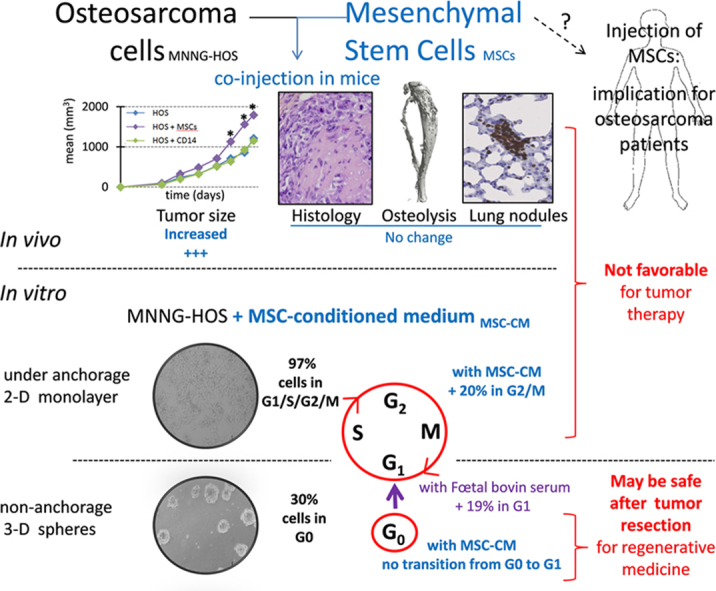
Facing a unique clinical case of osteosarcoma recurrence following autologous adipose-tissue transfer [30], we started to investigate the interactions between osteosarcoma and adipose tissue by using pre-clinical experiments [30], [64]. In the present report, we compared the interactions of MNNG-HOS cells-induced osteosarcoma with human ADSCs/MSCs and with human pre-osteoclasts. It is established that osteoclasts are involved in osteosarcoma progression and are believed to either enhance or suppress metastases [31], [32], [33]. In this study, pre-osteoclasts did not increase the tumor size and the lung metastasis. In contrast, ADSCs and MSCs increased the size of MNNG-HOS-induced tumors, but the metastasis process and rate of osteolysis were not exacerbated. Paracrine effects of ADSCs were investigated on osteosarcoma cells after culture in monolayer or oncospheres in order to observe the effects on proliferative or quiescent cell stages. The addition of 50% ADSC-conditioned medium significantly increased the in vitro proliferation of two osteosarcoma cell lines (MNNG-HOS and Saos-2), whereas it did not decrease the proportion of cells in G0 phase. These results suggest that ADSCs/MSCs may be safe in reconstructive surgery after bone tumor resection and not involved in the risk of local recurrence. However, ADSCs/MSCs do not appear to be good candidates for tumor-targeted cell therapy in osteosarcoma, given their enhancing effects on tumor progression.
2. Materials and methods
2.1. Ethics statement
Adipose tissue samples were obtained from patients who underwent abdominal liposuction in the plastic surgery department of Nantes University Hospital (France). Bone marrow aspirates were obtained from patients during orthopaedic surgical procedures in Tours University Hospital (France). Blood samples were obtained from the “Etablissement Français du Sang” in Nantes. Oral consent was obtained from informed patients in accordance with French law (Art. L. 1245-2 of the French public health code, Law no. 2004-800 of 6 August 2004, Official Journal of 7 August 2004). The donors had no significant medical history.
Experiments involving animals were conducted in accordance with French guidelines (named “Charte nationale portant sur l'éthique de l'expérimentation animale” by the French ethics committee) and were approved by the regional committee on animal ethics named CEEA.PdL.06, with project authorization number 2013.4.
2.2. Cell lines and culture conditions
2.2.1. Osteosarcoma cell lines
MNNG-HOS and Saos-2 cells were purchased from the American Type Culture Collection (ATCC numbers CRL-1547 and HTB-85 respectively, Manassas, VA, USA). The cells were cultured in Minimum Essential Medium alpha with nucleosides and 1 g/L D-Glucose (Gibco® MEM α; Life technologies, Saint Aubin, France) and supplemented with 10% fetal bovine serum (FBS, GE Healthcare, Vélizy-Villacoublay, France), at 37 °C in a humidified atmosphere (5% CO2/95% air). For culture under anchorage-independent conditions, medium was supplemented with 1.05% of methylcellulose (R&D Systems, Lille, France) and 2.5% FBS. MNNG-HOS cells were named LucF-HOS cells when they were modified to express the Enhanced Fluorescent Green Protein (EGFP) and firefly luciferase (LucF) genes as previously described [34].
2.2.2. Adipose- or bone marrow-derived stem cells
ADSCs were obtained from human fat samples which were removed using the Coleman's procedure [30], [35], [36], [37] and MSCs were obtained from human bone marrow aspirates [38]. From human fat or bone marrow samples, adherent cells were obtained and at passage 3, they were characterized. As previously described, [30], [39] flow cytometry analysis was performed to detect surface markers (CD105, CD90, CD75, CD45, CD34 and CD3) and their differentiation capacity towards osteogenic, adipogenic, chondrogenic or leiomyocyte lineages was assessed. ADSCs were transduced using EGFP-expressing lentiviral particles [34].
2.2.3. CD14 cells
They were obtained from human peripheral blood mononuclear cells and selected with CD14 Microbeads by MACS technology (Miltenyi Biotec, Paris, France) [40]. To induce pre-osteoclast differentiation, CD14+ monocytes were cultured in alpha-MEM containing 10% FBS, 25 ng/mL−1 of human macrophage colony stimulating factor (MCSF; from R&D Systems) and 100 ng/mL−1 of human RANKL (kindly provided by Amgen Inc., Thousand Oaks, USA) for 7 days.
2.2.4. Conditioned media
ADSCs and MSCs were cultured to near confluence with MEM α medium supplemented with 10% FBS, washed twice and cultured overnight in serum-free medium which was then collected and frozen (−20 °C), constituting ADSC- or MSC-conditioned medium (CM).
2.3. Osteosarcoma model
2.3.1. Tumor induction
Four-week-old female athymic mice (NMRI nu/nu; Elevages Janvier, Le Genest St Isle, France) were housed under pathogen-free conditions at the Experimental Therapy Unit (Faculty of Medicine, Nantes, France). The mice were anaesthetized by inhalation of an isoflurane-air mix (2% for induction and 0.5% for maintenance, 1 L/min) before any surgical manipulation and by intraperitoneal injection of a ketamine-xylazine mix (16 mg/kg and 66 mg/kg respectively) for bioluminescence measurements. Osteosarcoma development was induced into the tibial anterior muscle, by injection of 2×106 MNNG-HOS cells, alone or with ADSCs, MSCs or pre-osteoclasts at indicated ratios. Tumor volume was calculated with the formula (l2xL)/2 where l and L represent the smallest and largest diameter respectively.
2.3.2. Histology analysis
Tumor samples were fixed in 10% buffered formaldehyde and then decalcified in 4% EDTA 0.2% paraformaldehyde (PFA, pH 7.4) buffer for 4 weeks. After embedding in paraffin wax, 5 μm-thick sections were stained with Haematoxylin–Eosin–Safran (HES). Human nucleus detection was performed using a digoxin-labeled human locked nucleic acid Alu probe (Exiqon, Vedbaek, Denmark) as described previously [10]. Briefly, 70 nM Alu was hybridized on histological sections following DNA denaturation, in a buffer containing 4 X SSC (S6639, Sigma Aldrich, St. Quentin Fallavier, France), 50% deionized formamide, 1 X Denhardt's solution, 5% dextran sulfate and 100 µg/mL Salmon sperm DNA, for 19 h at 56 °C. Finally, the Alu probe was detected by peroxidase-based immunohisto-chemical procedure. For Tartrate-Resistant Acid Phosphatase (TRAP) detection slides were incubated 1 h in a 1 mg ml−1 naphthol AS-TR phosphate, 60 mmol l−1N,N-dimethylformamide, 100 mmol l−1 sodium tartrate and 1 mg ml−1 Fast Red TR salt solution (Sigma Aldrich, Saint Quentin Fallavier, France) and counterstained with haematoxylin. EGFP detection was observed on frozen sections with fluorescence microscopy directly or after immunostaining using mouse monoclonal to GFP primary antibody (Abcam, Paris, France) and Alexa Fluor 594 goat anti-mouse IgG (Life Technologies, Saint-Aubin, France).
2.3.3. Lung metastasis detection
Lungs were systematically observed for macroscopic nodules at necropsy. Macroscopic detection was completed by bioluminescence detection for tumor models induced with luciferase-expressing MNNG-HOS cells. The animals were placed individually in an induction chamber, and anaesthesia was induced with 3% isoflurane in oxygen. Then, mice were intra-peritoneally injected with 3 mg D-Luciferin (Interchim, Montluçon, France) in 250 µl of water, based on 25 g weight. After 7 min, mice were sacrificed for quick extraction of the lungs, which were placed into a photon Imager NightOWL LB 981 (Berthold technologies, Thoiry, France). Bioluminescence acquisition was performed for two 1.5 min. The BLI is expressed as photons per pixel per second after background subtraction.
2.3.4. Micro-computed tomography (µCT)
Mouse tibiae were fixed in 10% buffered formaldehyde and scanned with a high resolution X-ray micro–computed tomography Skyscan 1076 (Skyscan, Kontich, Belgium) using the following parameters: pixel size 18 µm, 50 kV, 0.5 mm Al filter, for 15 min. The 3D reconstruction was analyzed using the NRecon software. Bone parameters following the bone ASBMR nomenclature were quantified using the CTan software (Skyscan) and performed on 2 mm of tibia from the fibula insertion point.
2.4. Cell viability
Three thousand cells per well were seeded into 96-well plates and after the appropriate culture period, WST-1 reagent (Roche Diagnostics, Meylan, France) was added to each well and incubated for 15 h at 37 °C; absorbance was read at 490 nm. During the culture period, medium was supplemented with fat-CM or ADSC-CM or recombinant human leptin as indicated (R&D Systems) or with an antibody directed against the subunit gp130 of IL-6 family receptor (10 µg/mL; clone BK5 from Diaclone, Besançon, France) as described [41].
2.5. Cell cycle analysis
Subconfluent cells were treated as indicated and labeled with propidium iodide [42]. FITC-coupled mouse anti-human Ki-67 antibody or irrelevant isotype antibody for control (BD Pharmingen, France) was eventually added before DNA staining with propidium iodide. Cell cycle distribution based on 2n or 4n DNA content was analyzed by flow cytometry (Cytomics FC500; Beckman Coulter, France) and 20,000 events were analyzed with MultiCycle AV Software, Windows version (Phoenix Flow System; San Diego, CA, USA) and CXP Analysis software version 2.2 (Beckman Coulter).
2.6. Multiplex assay
Quantification of soluble factors listed in Fig. 3c was performed using the Luminex technology (Bio-Plex Pro Assays, Bio-Rad, Marnes la Coquette, France) according to the manufacturer's instructions.
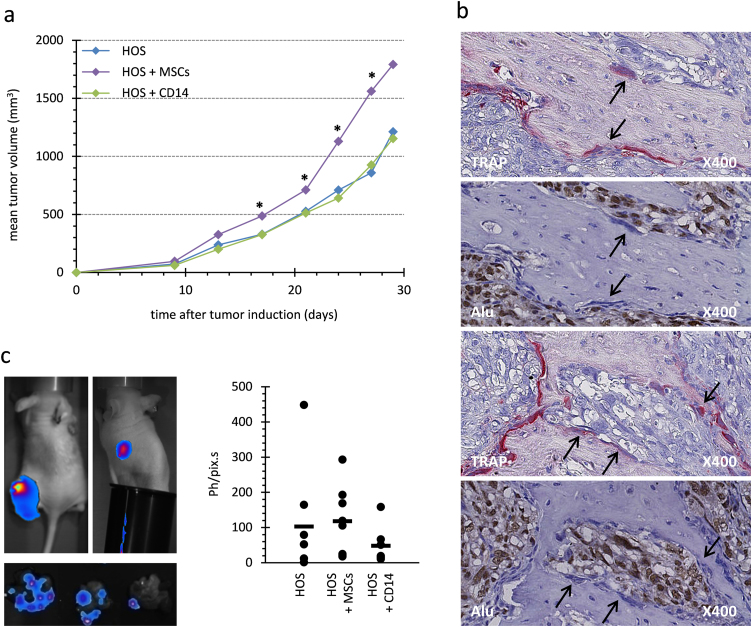
Fig. 3.
Co-injection of MNNG-HOS cells with MSCs or pre-osteoclasts in athymic mouse. (a) Evolution of median tumor volumes is reported for each group of mice (n=6). The HOS groups were injected with 2.106 MNNG-HOS cells, while the groups HOS+MSCs and HOS+CD14 were injected with 2.106 MNNG-HOS cells plus 1 million MSCs or RANKL-MCSF-activated CD14 cells, respectively. Significant differences between HOS+MSCs and HOS groups are indicated by single stars for p<0.05. (b) TRAP staining and Alu-sequence hybridization were performed on serial sections of tumors obtained 34 days after HOS+CD14 injection. Arrows indicate potential osteoclasts that were TRAP positive (red staining) but negative for Alu hybridization (nuclei stained in blue). In contrast numerous Alu-positive nuclei corresponding to MNNG-HOS cells were stained in brown. (c) Luciferase-activity was detected at day 34 after cell injections. One representative image of bioluminescence in vivo detection is shown for primary-tumor sites (top left panel), for secondary-tumor sites at lungs using a cache for primary sites (top right panel) and for excised lung lobes (low panel). The quantification of ex vivo luciferase activity of lung lobes is expressed as photons per pixel per second (Ph/pix s) after subtraction of photon noise. Quantification is showed for each animal (point) and the mean value is showed for each group (bar). No significant differences were observed between groups.
2.7. Reverse Transcription and Quantitative PCR
RNA was extracted using NucleoSpin RNA II (Machery-Nagel, Düren, Germany). Reverse transcription (RT) was performed using 2 µg of total RNA and ThermoScript RT (Invitrogen Life Technologies). Then 20 ng of cDNA were amplified using the IQ SYBR Green Supermix (Bio-Rad) with primers. Gene names and primer sequences are indicated in Table 1. Quantitative analysis was performed with the iCycler iQ Real-time PCR Detection System (Bio-Rad). Relative fold change of gene expression was calculated following the delta delta Ct method [43]. The reference genes ACTB and GAPDH were used for normalization.
Table 1.
List of genes analyzed by real time RT-PCR. Genes are presented with official gene symbols, corresponding full name and other used name. Forward and reverse primer sequences used to perform the analyses are indicated.
| Official Symbol | Official full name;Other name | Sense primer Antisense primer |
|---|---|---|
| ACTB | Actin beta | CCAACCGCGAGAAGATGA CCAGAGGCGTACAGGGATAG |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | TGGGTGTGAACCATGAGAAGTATG GGTGCAGGAGGCATTGCT |
| POU5F1 | POU class 5 homeobox 1; OCT4 | CAATTTGCCAAGCTCCTGA AGATGGTCGTTTGGCTGAAT |
| SOX2 | SRY (sex determining region Y)-box 2 | GTATCAGGAGTTGTCAAGGCAGAG TCCTAGTCTTAAAGAGGCAGCAAAC |
| NANOG | NanogHomeobox | ATGCCTCACACGGAGACTGT AAGTGGGTTGTTTGCCTTTG |
| RUNX2 | Runt-related transcription factor 2; CBFA1 | GTGCCTAGGCGCATTTCA GCTCTTCTTACTGAGAGTGGAAGG |
| SOX9 | SRY (sex determining region Y)-box 9 | GTACCCGCACTTGCACAAC TCGCTCTCGTTCAGAAGTCTC |
| PPARG | Peroxisome proliferator-activated receptor gamma | GACCTGAAACTTCAAGAGTACCAAA TGAGGCTTATTGTAGAGCTGAGTC |
| FABP4 | Fatty acid binding protein 4 | CCTTTAAAAATACTGAGATTTCCTTCA GGACACCCCCATCTAAGGTT |
| MYC | Myelocytomatosis viral oncogene homolog | CACCAGCAGCGACTCTGA GATCCAGACTCTGACCTTTTGC |
2.8. Statistical analysis
GraphPad InStat v3.02 software (La Jolla, CA, USA) was used. In vivo experimentation results were analyzed with the unpaired nonparametric method and Dunn's multiple comparisons following the Kruskal–Wallis test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. MSC-like cells increase MNNG-HOS osteosarcoma growth without exacerbation of osteolytic lesions and lung metastases
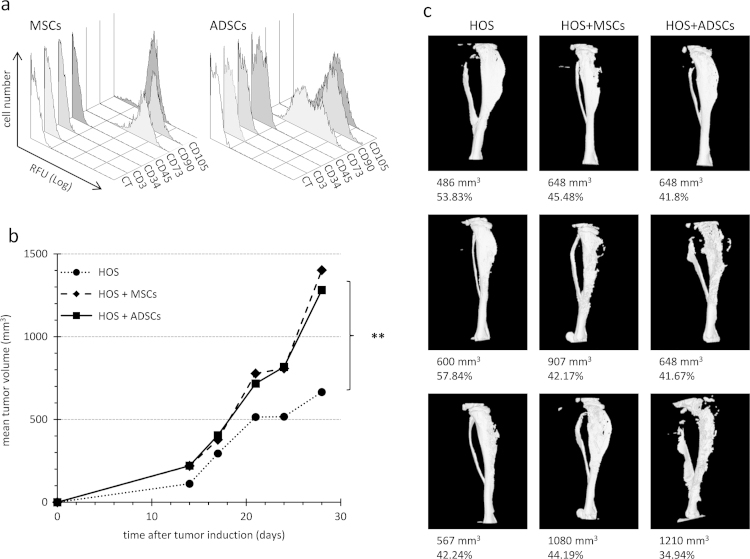
In order to know whether MSC-like cells can affect human osteosarcoma growth, they were co-injected with MNNG-HOS cells in mice. Adherent cells derived from human bone marrow or adipose tissue displayed similar phenotype, being CD3, CD34 and CD45 negative and CD73, CD90 and CD105 positive (Fig 1a). For meeting the minimal criteria to define mesenchymal stem cells [44], these cells were differentiated into at least three different lineages as previously shown [30], [39]. MSCs or ADSCs have induced a similar increase in the growth of MNNG-HOS-induced tumors when they were co-injected in comparison with MNNG-HOS cells alone (Fig. 1b). Furthermore we observed that a higher dose of ADSCs induced a stronger growth of tumors (Fig. 2a). It was shown that osteolysis enhances osteosarcoma growth in some murine models [33], [45]. Therefore, human osteoclast precursors, obtained by stimulating CD14 positive cells with MCSF and RANKL were also co-injected with MNNG-HOS cells. In contrast to human MSCs, osteoclast precursors (CD14 positive cells) did not change tumor growth (Fig. 3a). ADSCs, MSCs or CD14-positive cells did not induce any tumor development when injected alone in mice.
Fig. 1.
Co-injection of MNNG-HOS cells with ADSCs and MSCs in athymic mouse. (a) ADSCs and MSCs were characterized by flow cytometry analysis. After the third passage in culture, cells were incubated with fluorescent-coupled antibody directed against the indicated clusters of differentiation (CD) and compared to unlabelled cells (CT). (b) Mean tumor volume evolution is presented for each group of mice (n=8). HOS group was induced with 2.106 MNNG-HOS cells, while the groups HOS+ADSCs and HOS+MSCs were injected with 2.106 MNNG-HOS cells plus 5.105 ADSCs or MSCs, respectively. Significant differences are indicated only for HOS groups with other groups at day 30 (**: p<0.01). (c) Microscanner images of tibiae bearing tumors are shown. Tumor volume (mm3) and specific bone volume (%) are indicated.
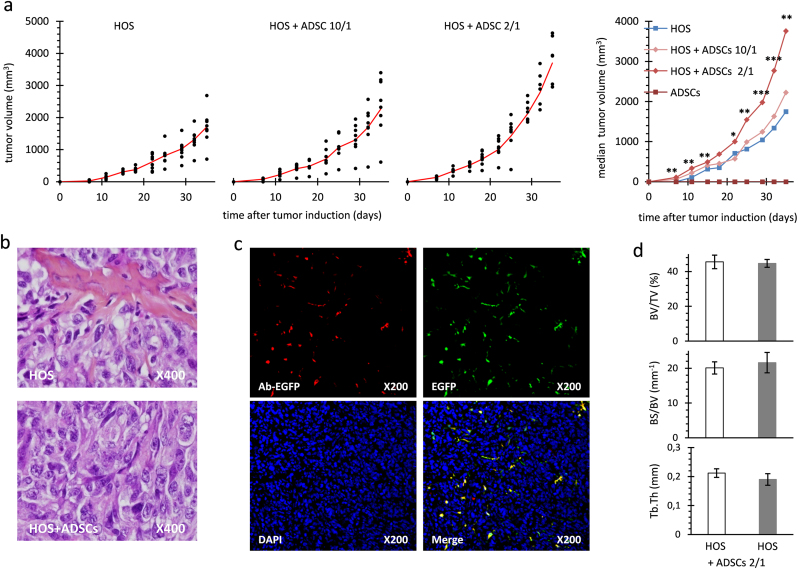
Fig. 2.
Co-injection of MNNG-HOS cells with ADSCs at ratio 10/1 or 2/1 in athymic mouse. (a) Tumor progression is reported for three groups of mice (n=8) injected with 2 million MNNG-HOS cells either alone, or with 1 million or 0.2 million of ADSCs, named HOS, HOS+ADSCs 10/1, and HOS+ADSCs 2/1 respectively. Individual tumor volumes are represented as dots and mean tumor volumes as red lines. Mean tumor volumes are presented for the three groups injected with MNNG-HOS cells and for the group injected with 1 million ADSCs alone. Significant differences between the MNNG-HOS and the HOS+ADSCs 2/1 groups are indicated (*: p<0.05; **: p<0.01; ***: p<0.001). (b) Hematoxylin-Eosin-Safran staining on tumor sections revealed undifferentiated sarcomas. (c) EGFP-expressing ADSCs were detected at day 10 post-tumor induction. EGFP was detected either indirectly by immunofluorescence (red, Ac-EGFP) or by direct fluorescence (green, EGFP). Nuclei were stained with DAPI (blue). Images (red, green, blue) were combined using ImageJ software (Merge). Original magnifications are indicated. (d) Tibia remodelling was analyzed when mean tumor volume reached 3000 mm3 in each group, corresponding to day 45 or 35 after tumor induction for HOS and HOS+ADSCs 2/1 groups, respectively. Mean parameters and SD are shown for percent bone volume (BV/TV in %), bone surface/volume ratio (BS/BV) and trabecular thickness (Tb.Th).
As depicted in Fig. 2b, histology analysis showed very similar characteristics with undifferentiated connective tissues in all groups. When EGFP-expressing ADSCs were co-injected with MNNG-HOS cells in mice, EGFP was detected within the tumor sections at day 10 post-tumor induction (Fig. 2c), but was no longer detected at day 24. Starting with 33% of ADSCs, the MNNG-HOS tumors contained less than 5% of ADSC at day 10 and none at day 24 post-tumor induction. Therefore, the tumor mass grew mostly through the MNNG-HOS cell proliferation and was not due to ADSC proliferation.
Bone morphometry parameters for the tibia in contact to tumors appeared very similar in all groups (Figs. 1c and 2d). In mice that were co-injected with human pre-osteoclasts, Alu-sequence-hybridization and TRAP staining were performed on serial sections of mouse tibia bearing osteosarcoma at day 30 post-tumor induction (Fig. 3b). Alu-sequence-hybridization signal identified the human tumor cells, but did not co-localize with TRAP-positive osteoclasts, indicating that no functional osteoclast may have derived from human CD14 positive cells. This result correlated with the fact that no increase in osteolysis was observed in MNNG-HOS+CD14 group (data not shown).
Firefly luciferase-expressing MNNG-HOS cells were used to reveal lung metastases by bioluminescence. During the entire animal imaging, strong signals at the primary-tumor injection sites were detected, but signals in lungs were rarely detected (Fig. 3c). Imaging of excised lung lobes detected metastases in all animals, even though only half of the mice were identified with macroscopic lung metastases. Bioluminescence quantification of lung metastases did not show significant differences between the groups injected with tumor cells alone or those with MSCs or osteoclast precursors (Fig. 3c).
In a manner very similar to what was observed with the injection of human adipose tissue, MSCs and ADSCs increased the tumor growth of primary human osteosarcoma, whereas human osteoclast precursors did not. The metastasis process and the massive osteolysis that is always observed on mouse tibia bearing MNNG-HOS-induced tumors were not exacerbated by the co-injection of human MSC-like cells or pre-osteoclasts.
3.2. Paracrine effect of MSC-like cells on proliferative or quiescent osteosarcoma cells
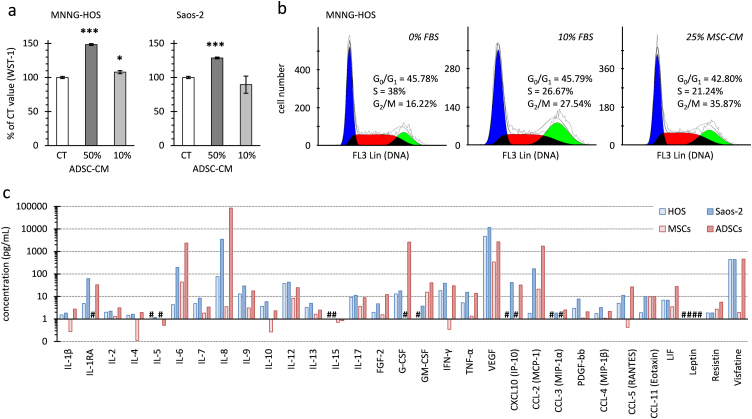
MSC-like cells may support osteosarcoma growth through secreted growth factors and cytokines. Supporting this hypothesis, the addition of 50% ADSC-conditioned medium (CM) without FBS complementation significantly increased the in vitro proliferation of two osteosarcoma cell lines (MNNG-HOS and Saos-2) as measured by trypan blue counting or WST-1 assay (Fig. 4a). MSC-CM also activated the in vitro proliferation of osteosarcoma cell lines. As deciphered by cell cycle analysis (Fig. 4b), MSC-CM increased the proportion of MNNG-HOS cells in the G2/M phase similarly to 10% FBS complementation in comparison to 0% FBS. Among 29 cytokines/growth factors measured by multiplex assay (Fig. 4c), we did not identify a paracrine mediator that would be present in both MSC- and ADSC-CM, but that would not be an autocrine mediator secreted by MNNG-HOS and Saos-2 cells. Vascular endothelial growth factor (VEGF) concentrations were greater in osteosarcoma cell-CM than in medium conditioned by MSCs. Interleukin-8 and -6 (IL-8 and IL-6) concentrations were higher in medium conditioned by ADSCs than in osteosarcoma cell-CM or MSC-CM. Similarly to what was observed using adipose tissue-CM [64], the osteosarcoma cell proliferation induced by ADSC-CM was not prevented by inhibition of IL-6 or IL-8 signaling with neutralizing antibody directed against the gp130 subunit or the chemokine (C-X-C motif) receptor 1/2 respectively (data not shown).
Fig. 4.
MSC-like cell secreted factors on proliferation of osteosaroma cells in vitro. (a) Mitochondrial activity of MNNG-HOS or Saos-2 cells was measured after 24 h in medium without FBS (CT) or supplemented with ADSC-conditioned medium representing 10% or 50% of the total volume. Results are the means of 3 wells and are presented as percentage of control (CT) with standard deviations. Significant differences between CT and ADSC-CM are indicated (*: p<0.05; ***: p<0.001). (b) Cell cycle analysis of MNNG-HOS cells was measured after 24 h in medium not supplemented (0%) or supplemented with FBS (10%) or MSC-CM (25%). The histograms represent cells numbers in cell phases. Because only 3-2% of cells were identified in the sub-G0 phase, only the proportion of cells in the G0/G1, S and G2/M phases are indicated. (c) Quantification of different cytokines or growth factors using multiplex immunoassay in medium conditioned by MNNG-HOS, Saos-2 cells, ADSCs or MSCs. Interleukins (IL), chemokine ligands (CCL and CXCL), growth factors (fibroblast GF-2, vascular endothelial GF-A, platelet-derived GF-bb, tumor necrosis factor alpha, interferon gamma, leukemia inhibitory factor) and adipokines (leptin, resistin, visfatin) were measured. #: not detected.
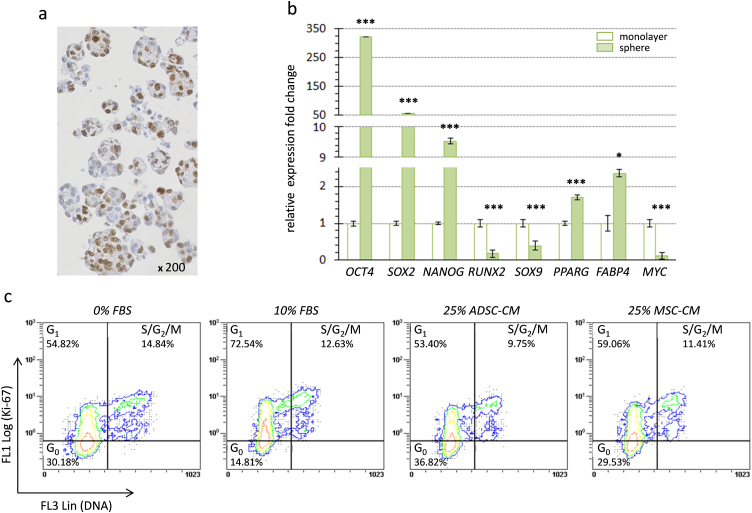
Considering that MSC-like cells are only used following chemotherapy and tumor resection for bone or soft tissue repair, it may be of interest to test their paracrine effect on quiescent osteosarcoma cells rather than on proliferating ones. To this aim, MNNG-HOS cells were cultured without anchorage, inducing the formation of oncospheres (Fig. 5a), as done to isolate cancer stem cells from osteosarcoma biopsies [46] or to induce a cancer stem cell-like phenotype [47]. As expected [48], we observed a significant enhanced expression of embryonic stem-cell specific transcription factors such as OCT4 (POU5F1; >300 fold), SOX2 (>50 fold) and NANOG (>9 fold) for MNNG-HOS cells cultured in oncospheres compared to those cultured in monolayers (Fig. 5b). A concomitant down-regulation of osteogenic and chondrogenic specific genes (0.33-fold for RUNX2 and 0.4-fold for SOX9) was noted, while expression of adipogenic specific genes were slightly increased (1.7-fold for PPARG and 2.4-fold for FABP4). Furthermore, MNNG-HOS cells cultured in oncospheres slowed down their proliferation as shown by the decrease of MYC expression (Fig. 5b) and by the low proportion of MNNG-HOS cells that were detected in S/G2/M phases (<15%) (Fig. 5c). Cell cycle analysis combining DNA and Ki-67 staining also revealed a high proportion (30%) of non-dividing cells corresponding to cells in G0 cell cycle phase in oncospheres. However, FBS complementation enabled part of G0 cells to enter G1 phase (2-fold decrease of G0 cells). In contrast ADSC- or MSC-CM did not decrease the proportion of cells in G0. These results indicate that ADSCs and MSCs, through secreted factors, may accelerate the cell cycle of proliferating osteosarcoma cells, but may not change the quiescent state of dormant tumor cells.
Fig. 5.
MSC-like cell secreted factors on quiescent osteosaroma cells in vitro. (a) Representative image of spheres obtained with MNNG-HOS cells cultured under an anchorage-independent condition. Immunohistochemistry detection of Ki-67 revealed a high proportion of proliferating cells (>50%). (b) Relative expression fold changes are presented for mRNA of MNNG-HOS cells cultured either under anchorage (monolayer) or non-anchorage conditions (spheres). Gene name symbols with corresponding full names are indicated in Table 1. Results are means of 3 samples and are presented with standard deviations. Significant differences between the anchorage and non-anchorage culture conditions are indicated (*: p<0.05; ***: p<0.001). (c) Dot plots present the % of MNNG-HOS cells in different cell cycle phases following DNA and Ki-67 detection by flow cytometry. MNNG-HOS cells cultured in oncosphere were incubated for 48 h in medium not supplemented (0% FBS) or supplemented with 10% FBS (10%) or 25% ADSC/MSC-CM. Results are representative of 3 independent experiments.
4. Discussion
We have previously shown that murine MSC-like cells increased tumor growth when they were co-injected with osteosarcoma cells in mice. In this study, we demonstrate that osteosarcoma growth is also supported by the co-injection of human MSC-like cells, either derived from adipose tissue or bone marrow, but not by osteoclast precursors. In tumors induced by the co-injection of MNNG-HOS cells and ADSCs, ADSCs were not identified at the end of in vivo experiments. Soluble factors produced by ADSC increased by up to 2-fold the in vitro proliferation of two different osteosarcoma cell lines (MMNG-HOS and Saos-2). These results indicate that ADSCs/MSCs may modulate the early MNNG-HOS tumor development, at least partially through paracrine effects as described for cancer-associated adipocytes and breast cancer [49] or adipose tissue and osteosarcoma [64]. It has been suggested that MSCs improve angiogenesis after an ischemic lesion [50], [51] or facilitate successful fat injection during surgery for breast augmentation or reconstruction [19]. By stimulating neovascularization, MSC-like cells may contribute to sarcoma growth [25], [44]. VEGF was identified in ADSC/MSC-conditioned medium but in concentrations similar to MMNG-HOS/Saos-2 cell-conditioned medium. In samples of MNNG-HOS-induced tumors which have otherwise overgrown due to MSC-like cells, we could not observe any change in VEGFA expression nor in tumor neovascularization (data not shown). Bian et al. [27] have reported that human MSCs promoted Saos-2 osteosarcoma growth but through Interleukin 6 (IL-6) secretion. In the present study, IL-6 and IL-8 were indeed detected in the ADSC-conditioned medium. Nonetheless, they were not identified as the molecules that could mediate enhanced proliferation of MMNG-HOS and Saos-2 cells. Similarly, basic fibroblast growth factor (FGF-2) did not enhance the proliferation of osteosarcoma cells in vitro (data not shown), despite the fact that a role for FGF-2 in tumor progression was suggested, especially in an original mouse model of osteosarcoma [52]. Other soluble factors that were not investigated in this study included metalloproteinase 11 which can favor tumor progression, collagen VI involved in the breast cancer-adipocyte interaction [53] and lysophosphatidic acids implicated in development of bone metastases [54]. All these factors may be implicated, possibly acting in combination, in osteosarcoma cell proliferation.
Preclinical studies have shown that adipocytes and MSCs play an important role in migration and dissemination of cancer cells [55], [56]. Concerning osteosarcoma, one publication has reported that human MSCs injected through the caudal vein of mice bearing Saos-2- induced an increase in tumor volume associated with more severe osteolytic lesions and a higher rate of pulmonary metastasis [29]. A similar pro-metastatic effect was observed with rat MSCs injected intravenously in rats bearing osteosarcoma [28], [57], while co-implantation of rat MSCs and osteosarcoma cells did not promote lung metastasis [28]. In the present study, we did not observe an aggravation of osteolytic lesions and lung metastases despite tumor growth increase following co-injection of ADSCs/MSCs with MNNG-HOS cells in mice. Because the metastasis process was not changed by ADSCs/MSCs injection in vivo, ADSC-derived soluble factors were not tested in in vitro migration assays of osteosarcoma cells.
Growth of osteosarcoma is supported by the bones microenvironment including the activity of osteoclasts. The role of osteoclasts was demonstrated in osteosarcoma models through osteolysis blockage which enhanced tumor regression or allowed tumor growth inhibition [33], [58]. However, Endo-Munoz et al. [31] have reported that the loss of osteoclasts in the early development of osteosarcoma is associated with increased potential for osteosarcoma cells to metastasize. Surprisingly in the present study, the osteosarcoma development including local growth, lung metastasis and osteolysis, were not changed by the early co-injection of pre-osteoclasts (MCSF and RANKL-stimulated CD14 positive cells) with MNNG-HOS cells in mice. This experiment did not confirm that osteoclasts could activate osteosarcoma growth or prevent the metastasis process while no mature human osteoclasts could be detected in vivo following co-injection.
MSC appear to have a dual nature, regarding their ability to promote tumor growth and metastasis or to suppress tumor progression [26]. The reasons for the discrepant actions of MSCs on tumor growth are under investigation but potentially may be attributed to differences in tumor models, the dose or timing of MSC injections, the animal host, or the inflammatory status of MSCs [59]. In view of the enhancing effect of ADSCs/MSCs on the proliferation of osteosarcoma cells both in vivo and in vitro, MSCs may not be good candidates for osteosarcoma-targeted cell therapy.
Nonetheless, the models of MSC injection in rodents bearing fast-growing tumors are unlikely useful for clinical application in reconstructive surgery following chemotherapy and/or tumor resection. Aanstoos et al. [60] have recently tested MSC injection after amputation in mice bearing osteosarcoma and showed that MSC injection at the surgical site did not promote pulmonary metastasis or local recurrence compared to no-MSC injection; however MSC intravenous injection induced a faster development of pulmonary metastasis. More than 20 years ago, Hernigou et al. supplemented bone allografts with autologous bone marrow from the iliac crest and then started to use nucleated cells concentrated from bone marrow aspirate. Following implantion of autologous concentrated bone marrow cells including MSCs into 1873 patients without malignancy, there was no excess of neoplastic events over the expected number in a normal population [61]. Similarly, no increased risk of neoplasia was reported in a cohort of more than 200 patients treated with MSCs for different regenerative medicine applications [62]. Furthermore, Hernigou et al. reported that the autologous concentrated bone marrow cell adjuvant therapy in 92 patients treated after bone tumor resection did not increase the risk of local tumor recurrence compared to control populations [63].
In the present study, the effect of ADSC-secreted factors were tested on dormant cancer cells that could be present following therapy. For this purpose, osteosarcoma cells were cultured in oncospheres to induce a cancer stem cell-like phenotype [47]. We observed that conditioned medium of ADSCs did not change the quiescent state of dormant osteosarcoma cells when they were cultured in oncospheres, while such medium accelerated the cell cycle of proliferating osteosarcoma cells. This result indicates that ADSC-secreted factors may not be involved in the local recurrence by activation of cancer stem cells. This result combined with those obtained with adipose tissue-secreted factors [64] is reassuring for the complementation of adipose tissue transfer with ADSCs in plastic reconstructive surgery, but further investigation using preclinical models which mimic quiescent state of osteosarcoma are still needed to warrant safe clinical use of ADSCs following osteosarcoma resection.
Grant support
This work was supported by INSERM, the “Ligue Contre le Cancer” (Equipe Labellisée LIGUE 2012; grant EL2012NCC/DH), the “Fédération Nationale Enfants et Santé” and the “Société Française de lutte contre les Cancers et les leucémies de l'Enfant et de l'Adolescent(“appel à projet 2013”).
Acknowledgments
The authors wish to thank Guylène Hamery and Yohann Allain from the Experimental Therapy Unit platform of the Faculty of Medicine (Nantes, France) for their technical assistance. We thank Céline Charrier, Séverine Battaglia, Régis Brion, Jérôme Amiaud (all from INSERM UMR957, Nantes, France) for their kind technical assistances.
References
- 1.Marina N., Gebhardt M., Teot L., Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 2.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J. Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luetke A., Meyers P.A., Lewis I., Juergens H. Osteosarcoma treatment-where do we stand? A state of the art review. Cancer Treat. Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Donati D., Colangeli M., Colangeli S., Di Bella C., Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin. Orthop. Relat. Res. 2008;466:459–465. doi: 10.1007/s11999-007-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernigou P., Delepine G., Goutallier D., Julieron A. Massive allografts sterilised by irradiation. Clinical results. J. Bone Jt. Surg. Br. 1993;75:904–913. doi: 10.1302/0301-620X.75B6.8245080. [DOI] [PubMed] [Google Scholar]
- 6.Bus M.P., Dijkstra P.D., van de Sande M.A., Taminiau A.H., Schreuder H.W., Jutte P.C. Intercalary allograft reconstructions following resection of primary bone tumors: a nationwide multicenter study. J. Bone Jt. Surg. Am. 2014;96:e26. doi: 10.2106/JBJS.M.00655. [DOI] [PubMed] [Google Scholar]
- 7.Hilven P.H., Bayliss L., Cosker T., Dijkstra P.D., Jutte P.C., Lahoda L.U. The vascularised fibular graft for limb salvage after bone tumour surgery: a multicentre study. Bone Jt. J. 2015;97:853–861. doi: 10.1302/0301-620X.97B6.34692. [DOI] [PubMed] [Google Scholar]
- 8.Zimel M.N., Cizik A.M., Rapp T.B., Weisstein J.S., Conrad E.U., 3rd Megaprosthesis versus Condyle-sparing intercalary allograft: distal femoral sarcoma. Clin. Orthop. Relat. Res. 2009;467:2813–2824. doi: 10.1007/s11999-009-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 10.Brennan M.A., Renaud A., Amiaud J., Rojewski M.T., Schrezenmeier H., Heymann D. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell. Res. Ther. 2014;5:114. doi: 10.1186/scrt504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari S., Bacci G., Picci P., Mercuri M., Briccoli A., Pinto D. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann. Oncol. 1997;8:765–771. doi: 10.1023/a:1008221713505. [DOI] [PubMed] [Google Scholar]
- 12.Gamblin A.L., Brennan M.A., Renaud A., Yagita H., Lezot F., Heymann D. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: the local implication of osteoclasts and macrophages. Biomaterials. 2014;35:9660–9667. doi: 10.1016/j.biomaterials.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Meyers P.A., Heller G., Healey J., Huvos A., Lane J., Marcove R. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J. Clin. Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Grisendi G., Spano C., D'Souza N., Rasini V., Veronesi E., Prapa M. Mesenchymal progenitors expressing TRAIL induce apoptosis in sarcomas. Stem Cells. 2015;33:859–869. doi: 10.1002/stem.1903. [DOI] [PubMed] [Google Scholar]
- 15.Mohr A., Lyons M., Deedigan L., Harte T., Shaw G., Howard L. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J. Cell. Mol. Med. 2008;12:2628–2643. doi: 10.1111/j.1582-4934.2008.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grisendi G., Bussolari R., Veronesi E., Piccinno S., Burns J.S., De Santis G. Understanding tumor-stroma interplays for targeted therapies by armed mesenchymal stromal progenitors: the Mesenkillers. Am. J. Cancer Res. 2011;1:787–805. [PMC free article] [PubMed] [Google Scholar]
- 17.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu F., Li J., Gao J., Ogawa R., Ou C., Yang B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast. Reconstr. Surg. 2009;124:1437–1446. doi: 10.1097/PRS.0b013e3181babbb6. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura K., Sato K., Aoi N., Kurita M., Hirohi T., Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterodimas A., de Faria J., Nicaretta B., Boriani F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthetic Surg. J. 2011;31:682–693. doi: 10.1177/1090820X11415976. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Cano R., Vranckx J.J., Lasso J.M., Calabrese C., Merck B., Milstein A.M. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur. J. Surg. Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 22.Trojahn Kolle S.F., Oliveri R.S., Glovinski P.V., Elberg J.J., Fischer-Nielsen A., Drzewiecki K.T. Importance of mesenchymal stem cells in autologous fat grafting: a systematic review of existing studies. J. Plast. Surg. Hand Surg. 2012;46:59–68. doi: 10.3109/2000656X.2012.668326. [DOI] [PubMed] [Google Scholar]
- 23.Yingbo Z., Daping Y. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann. Plast. Surg. 2012;68:111. doi: 10.1097/SAP.0b013e31822d9e04. [DOI] [PubMed] [Google Scholar]
- 24.Hsu W.K., Wang J.C., Liu N.Q., Krenek L., Zuk P.A., Hedrick M.H. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J. Bone Jt. Surg. Am. 2008;90:1043–1052. doi: 10.2106/JBJS.G.00292. [DOI] [PubMed] [Google Scholar]
- 25.Burns J.S., Safwat A., Grisendi G., Kassem M., Dominici M. Sarcomas as a mise en abyme of mesenchymal stem cells: exploiting interrelationships for cell mediated anticancer therapy. Cancer Lett. 2012;325:1–10. doi: 10.1016/j.canlet.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Klopp A.H., Gupta A., Spaeth E., Andreeff M., Marini F., 3rd Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bian Z.Y., Fan Q.M., Li G., Xu W.T., Tang T.T. Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci. 2010;101:2554–2560. doi: 10.1111/j.1349-7006.2010.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukamoto S., Honoki K., Fujii H., Tohma Y., Kido A., Mori T. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int. J. Oncol. 2012;40:163–169. doi: 10.3892/ijo.2011.1220. [DOI] [PubMed] [Google Scholar]
- 29.Xu W.T., Bian Z.Y., Fan Q.M., Li G., Tang T.T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009;281:32–41. doi: 10.1016/j.canlet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Perrot P., Rousseau J., Bouffaut A.L., Redini F., Cassagnau E., Deschaseaux F. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One. 2010;5:e10999. doi: 10.1371/journal.pone.0010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo-Munoz L., Cumming A., Rickwood D., Wilson D., Cueva C., Ng C. Loss of osteoclasts contributes to development of osteosarcoma pulmonary metastases. Cancer Res. 2010;70:7063–7072. doi: 10.1158/0008-5472.CAN-09-4291. [DOI] [PubMed] [Google Scholar]
- 32.Endo-Munoz L., Evdokiou A., Saunders N.A. The role of osteoclasts and tumour-associated macrophages in osteosarcoma metastasis. Biochim. Biophys. Acta. 1826;2012:434–442. doi: 10.1016/j.bbcan.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Heymann D., Ory B., Blanchard F., Heymann M.F., Coipeau P., Charrier C. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37:74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Rousseau J., Escriou V., Perrot P., Picarda G., Charrier C., Scherman D. Advantages of bioluminescence imaging to follow siRNA or chemotherapeutic treatments in osteosarcoma preclinical models. Cancer Gene Ther. 2010;17:387–397. doi: 10.1038/cgt.2009.89. [DOI] [PubMed] [Google Scholar]
- 35.Coleman S.R. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast. Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 36.Covarrubias P., Cardenas-Camarena L., Guerrerosantos J., Valenzuela L., Espejo I., Robles J.A. Evaluation of the histologic changes in the fat-grafted facial skin: clinical trial. Aesthetic Plast. Surg. 2013;37:778–783. doi: 10.1007/s00266-013-0126-0. [DOI] [PubMed] [Google Scholar]
- 37.Delay E., Garson S., Tousson G., Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthetic Surg. J. 2009;29:360–376. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Cordonnier T., Langonne A., Sohier J., Layrolle P., Rosset P., Sensebe L. Consistent osteoblastic differentiation of human mesenchymal stem cells with bone morphogenetic protein 4 and low serum. Tissue Eng. Part C. Methods. 2011;17:249–259. doi: 10.1089/ten.TEC.2010.0387. [DOI] [PubMed] [Google Scholar]
- 39.Brennan M.A., Renaud A., Gamblin A.L., D'Arros C., Nedellec S., Trichet V. 3D cell culture and osteogenic differentiation of human bone marrow stromal cells plated onto jet-sprayed or electrospun micro-fiber scaffolds. Biomed. Mater. 2015;10:045019. doi: 10.1088/1748-6041/10/4/045019. [DOI] [PubMed] [Google Scholar]
- 40.Duplomb L., Baud'huin M., Charrier C., Berreur M., Trichet V., Blanchard F. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149:3688–3697. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- 41.Chevalier S., Fourcin M., Robledo O., Wijdenes J., Pouplard-Barthelaix A., Gascan H. Interleukin-6 family of cytokines induced activation of different functional sites expressed by gp130 transducing protein. J. Biol. Chem. 1996;271:14764–14772. doi: 10.1074/jbc.271.25.14764. [DOI] [PubMed] [Google Scholar]
- 42.Gobin B., Moriceau G., Ory B., Charrier C., Brion R., Blanchard F. Imatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine models. PLoS One. 2014;9:e90795. doi: 10.1371/journal.pone.0090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau J., Escriou V., Lamoureux F., Brion R., Chesneau J., Battaglia S. Formulated siRNAs targeting Rankl prevent osteolysis and enhance chemotherapeutic response in osteosarcoma models. Journal of bone and mineral research: the official journal of the American. Soc. Bone Miner. Res. 2011;26:2452–2462. doi: 10.1002/jbmr.455. [DOI] [PubMed] [Google Scholar]
- 46.Gibbs C.P., Kukekov V.G., Reith J.D., Tchigrinova O., Suslov O.N., Scott E.W. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson H., Huelsmeyer M., Chun R., Young K.M., Friedrichs K., Argyle D.J. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Vet. – J. 2008;175:69–75. doi: 10.1016/j.tvjl.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Levings P.P., McGarry S.V., Currie T.P., Nickerson D.M., McClellan S., Ghivizzani S.C. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69:5648–5655. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bochet L., Lehuede C., Dauvillier S., Wang Y.Y., Dirat B., Laurent V. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 50.Carriere A., Ebrahimian T.G., Dehez S., Auge N., Joffre C., Andre M. Preconditioning by mitochondrial reactive oxygen species improves the proangiogenic potential of adipose-derived cells-based therapy. Arterioscler. Thromb. Vasc. Biol. 2009;29:1093–1099. doi: 10.1161/ATVBAHA.109.188318. [DOI] [PubMed] [Google Scholar]
- 51.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu T., Ishikawa T., Iwai S., Ueki A., Sugihara E., Onishi N. Fibroblast growth factor-2 is an important factor that maintains cellular immaturity and contributes to aggressiveness of osteosarcoma. Mol. Cancer Res. 2012;10:454–468. doi: 10.1158/1541-7786.MCR-11-0347. [DOI] [PubMed] [Google Scholar]
- 53.Motrescu E.R., Blaise S., Etique N., Messaddeq N., Chenard M.P., Stoll I. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27:6347–6355. doi: 10.1038/onc.2008.218. [DOI] [PubMed] [Google Scholar]
- 54.David M., Wannecq E., Descotes F., Jansen S., Deux B., Ribeiro J. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS One. 2010;5:e9741. doi: 10.1371/journal.pone.0009741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dirat B., Bochet L., Dabek M., Daviaud D., Dauvillier S., Majed B. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen-Ngoc K.V., Cheung K.J., Brenot A., Shamir E.R., Gray R.S., Hines W.C. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. USA. 2012;109:E2595–E2604. doi: 10.1073/pnas.1212834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P., Dong L., Yan K., Long H., Yang T.T., Dong M.Q. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol. Rep. 2013;30:1753–1761. doi: 10.3892/or.2013.2619. [DOI] [PubMed] [Google Scholar]
- 58.Lamoureux F., Richard P., Wittrant Y., Battaglia S., Pilet P., Trichet V. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 59.Waterman R.S., Henkle S.L., Betancourt A.M. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7:e45590. doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aanstoos M.E., Regan D.P., Rose R.J., Chubb L.S., Ehrhart N.P. Do Mesenchymal Stromal Cells Influence Microscopic Residual or Metastatic Osteosarcoma in a Murine Model? Clin. Orthop. Relat. Res. 2015 doi: 10.1007/s11999-015-4362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernigou P., Homma Y., Flouzat-Lachaniette C.H., Poignard A., Chevallier N., Rouard H. Cancer risk is not increased in patients treated for orthopaedic diseases with autologous bone marrow cell concentrate. J. Bone Jt. Surg. Am. 2013;95:2215–2221. doi: 10.2106/JBJS.M.00261. [DOI] [PubMed] [Google Scholar]
- 62.Centeno C.J., Schultz J.R., Cheever M., Freeman M., Faulkner S., Robinson B. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr. Stem Cell. Res. Ther. 2011;6:368–378. doi: 10.2174/157488811797904371. [DOI] [PubMed] [Google Scholar]
- 63.Hernigou P., Flouzat Lachaniette C.H., Delambre J., Chevallier N., Rouard H. Regenerative therapy with mesenchymal stem cells at the site of malignant primary bone tumour resection: what are the risks of early or late local recurrence? Int. Orthop. 2014;38:1825–1835. doi: 10.1007/s00264-014-2384-0. [DOI] [PubMed] [Google Scholar]
- 64.Avril P., Duteille F., Ridel P., Heymann M.F., De Pinieux G., Gouin F., Rédini F., Blanchard F., Heymann D., Trichet V., Perrot P. Opposite effects of soluble factors secreted by adipose tissue on proliferating and quiescent osteosarcoma cells. Plast. Reconstr. Surg. 2015 doi: 10.1097/01.prs.0000479989.88114.8b. (in press) [DOI] [PubMed] [Google Scholar]