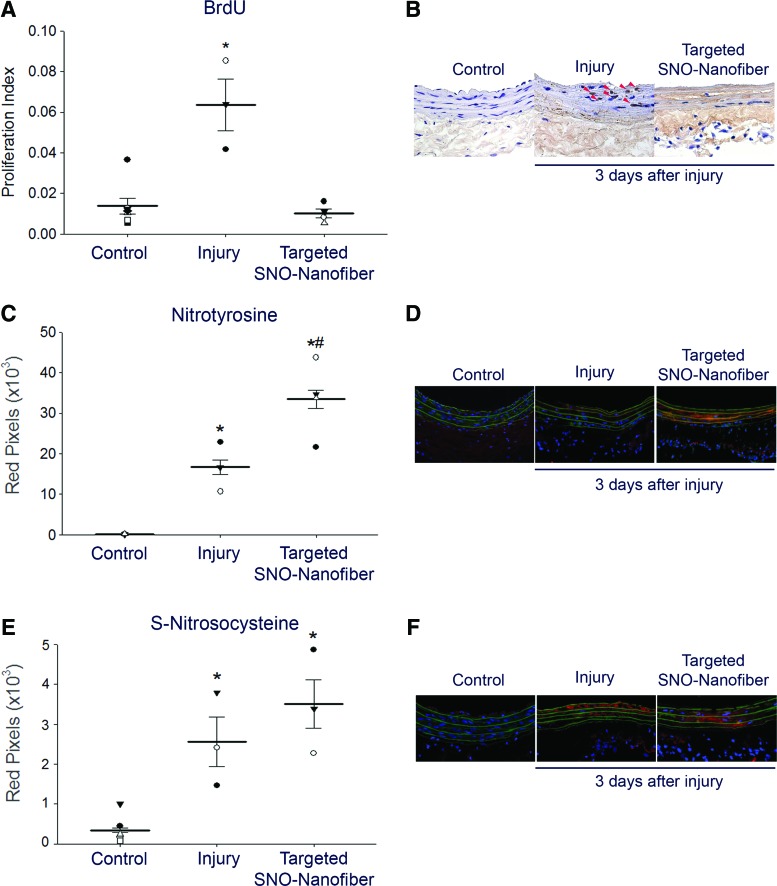
FIG. 5.
In vivo local effect of targeted SNO nanofibers on the vascular wall. The carotid artery balloon injury model was performed with or without tail vein administration of 2.5 mg targeted SNO nanofiber. At 24 and 1 h before sacrifice, a bromodeoxyuridine (BrdU) solution (100 mg/ml) was administered via intraperitoneal injection to label rapidly proliferating cells. At 3 days, carotid arteries were harvested for analysis. (A) Proliferative index (ratio of BrdU+ cells over total cells). N = 3 for injury alone, N = 4 for targeted SNO nanofiber, and N = 7 for uninjured control. Each data point represents one rat as the average of 10–20 technical replicates. *p < 0.05 compared with control and targeted SNO nanofibers. (B) Representative cross sections of the immunohistochemical staining using a 20× objective. Red arrowheads show BrdU-positive cells. (C) Immunofluorescence quantification for nitrotyrosine staining. N = 3 for injury alone, N = 4 for targeted SNO nanofiber, and N = 4 for uninjured control. Each data point represents one rat as the average of 10–20 technical replicates. *p < 0.05 compared with control and #p < 0.05 compared with injury alone. (D) Representative cross sections of immunofluorescence staining for nitrotyrosine (red) using a 20× objective. Green = elastic lamina; blue = nuclei. (E) Immunofluorescence quantification for S-nitrosocysteine staining. N = 3 for injury alone, N = 4 for targeted SNO nanofiber, and N = 7 for uninjured control. Each data point represents one rat as the average of 10–20 technical replicates. *p < 0.05 compared with control. (F) Representative cross sections of immunofluorescence staining for S-nitrosocysteine (red) using a 20× objective. Green = elastic lamina; blue = nuclei. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars