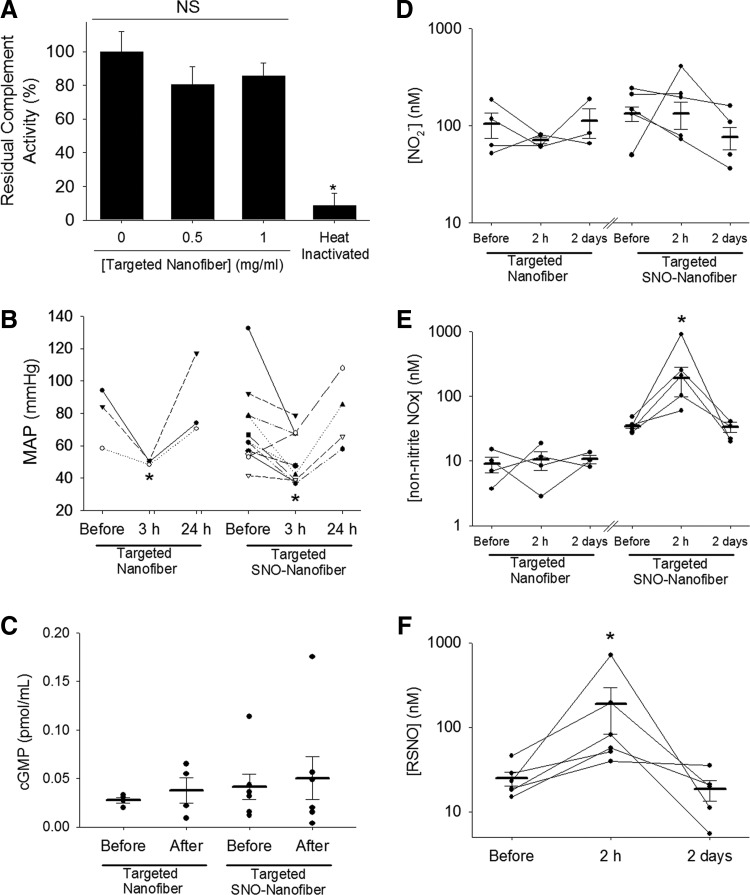
FIG. 7.
Systemic effects of the targeted SNO nanofibers. (A) Functional complement activation assay shows that the targeted SNO nanofibers do not activate the complement in human serum ex vivo. Residual compliment activity was measured after incubation with and without targeted nanofibers. Total depletion was achieved by heat inactivation of serum as a control. N = 3, *p < 0.05 versus nonheat treated. NS, nonsignificant. (B) MAP was measured before surgery and 3 h and 24 h after surgery and administration of 2.5 mg of either the targeted nanofiber or the targeted SNO nanofiber. N = 3 for targeted nanofiber, N = 4–10 for the targeted SNO nanofiber. *p < 0.05 with respect to MAP before surgery. (C) cGMP levels were measured in plasma before surgery and 2 hours after surgery and administration of 2.5 mg of either the targeted nanofiber or the targeted SNO nanofiber. N = 4 for targeted nanofiber, N = 6 for targeted SNO nanofiber. p = NS. Plasma reactive nitrogen species (NOx) were measured for (D) nitrite levels, (E) non-nitrite NOx, and (F) RSNO. Blood was collected before surgery and 2 h and 2 days after surgery and administration of 2.5 mg of either the targeted nanofiber or the targeted SNO nanofiber via chemiluminescent detection. N = 3–4 for targeted nanofiber, N = 4–5 for the targeted SNO nanofiber. *p < 0.05 with respect to levels before surgery. cGMP, cyclic guanosine monophosphate; MAP, mean arterial pressure.