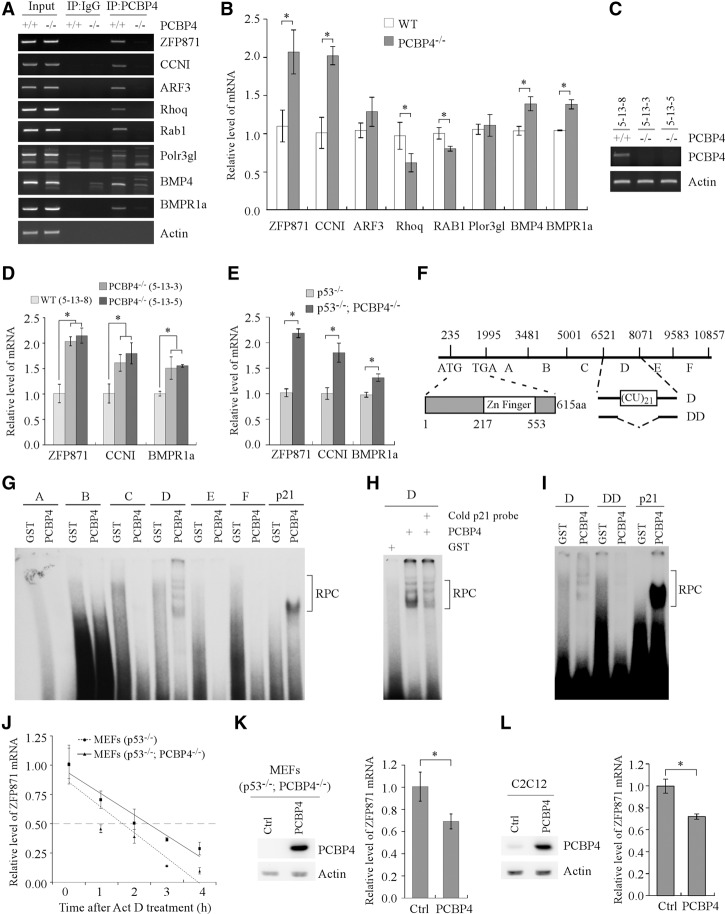
Figure 3.
Identification of ZFP871 as a target of PCBP4. (A) Interaction of PCBP4 protein with its target transcripts. Rabbit anti-PCBP4 antibody was used to immunoprecipitate PCBP4–RNA complexes from wild-type and PCBP4−/− MEFs along with IgG as a control. The binding of PCBP4 to the transcripts was measured by RT–PCR. Actin was used as a negative control. (B) qRT–PCR was performed to measure the level of PCBP4 target transcripts in wild-type and PCBP4−/− MEFs. Data are presented as mean ± SD normalized to actin mRNA from three separate experiments. (*) P < 0.05. (C) Generation of one wild-type and two PCBP4−/− MEFs from the same litter. (D) The level of ZFP871, CCNI, and BMPR1a transcripts in one wild-type and two PCBP4−/− MEFs shown in C was measured by qRT–PCR. (E) The level of ZFP871, CCNI, and BMPR1a transcripts in p53−/− and p53−/−; PCBP4−/− MEFs was measured by qRT–PCR. (F) Schematic presentation of the ZFP871 transcript, the predicted zinc finger domain in the ZFP871 protein, and the location of fragments A–F along with a CU-rich element in the ZFP871 3′ UTR. (G) The PCBP4 protein binds to probe D (nucleotides 6521–8070) but not probe A (nucleotides 1995–3480), B (nucleotides 3481–5000), C (nucleotides 5001–6520), E (nucleotides 8071–9582), or F (nucleotides 9583–10,857). RNA electrophoretic mobility shift assay (REMSA) was performed by mixing a 32P-labeled RNA probe with recombinant glutathione S-transferase (GST) or GST-fused PCBP4 protein. The binding of PCBP4 to the p21 3′ UTR was used as a positive control. The bracket indicates RNA–protein complexes (RPC). (H) REMSA competition assay was performed by adding an excess amount of unlabeled p21 cold probe to a reaction mixture containing the PCBP4 protein and 32P-labeled probe D. (I) REMSA was performed with probe DD, which lacks the CU-rich element (nucleotides 7324–7365). (J) The half-life of the ZFP871 transcript was measured by qRT–PCR in p53−/− and p53−/−; PCBP4−/− MEFs treated with 5 µg/mL actinomycin (Act D), an inhibitor of transcription. Data are presented as mean ± SD normalized to actin mRNA from three separate experiments. (K, left panel) The PCBP4 protein was measured by Western blot analysis in p53−/−; PCBP4−/− MEFs transiently transfected with pcDNA3 or pcDNA3-PCBP4. (Right panel) The level of ZFP871 transcript was measured by qRT–PCR in p53−/−; PCBP4−/− MEFs treated as in the left panel. Data are presented as mean ± SD after being normalized to actin mRNA from three separate experiments. P < 0.05. (L) The experiments were performed as in K except that C2C12 cells were used.