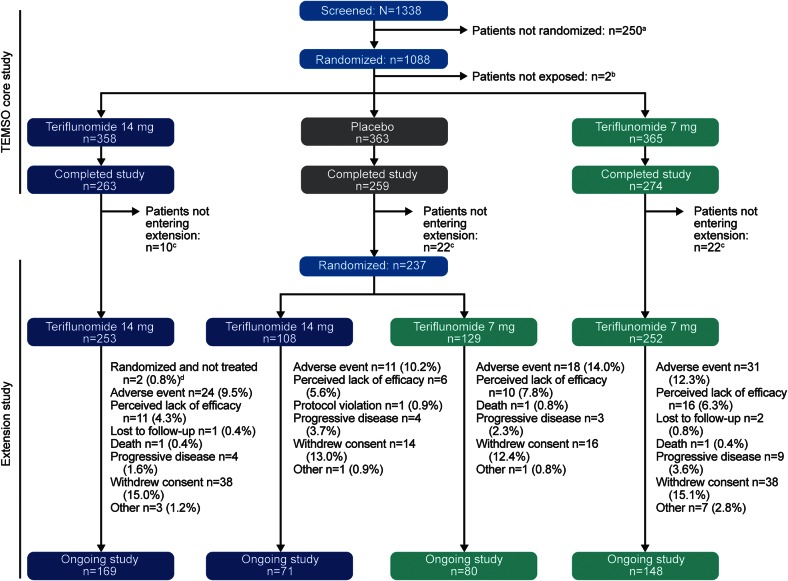
Figure 1. Patient disposition in Teriflunomide Multiple Sclerosis Oral (TEMSO) and the TEMSO extension study.
a The major reason for screening failure was failure to meet inclusion criteria (n = 155 [62.0%]); b 2 patients (1 each in the 14-mg and 7-mg teriflunomide groups) were not exposed to study medication because of protocol violation; c did not give consent to continue in extension study; d 2 patients in the 14-mg/14-mg group were randomized but not treated: 1 did not wish to continue and the other 1 prematurely discontinued study treatment and did not take any double-blind medication in the extension.