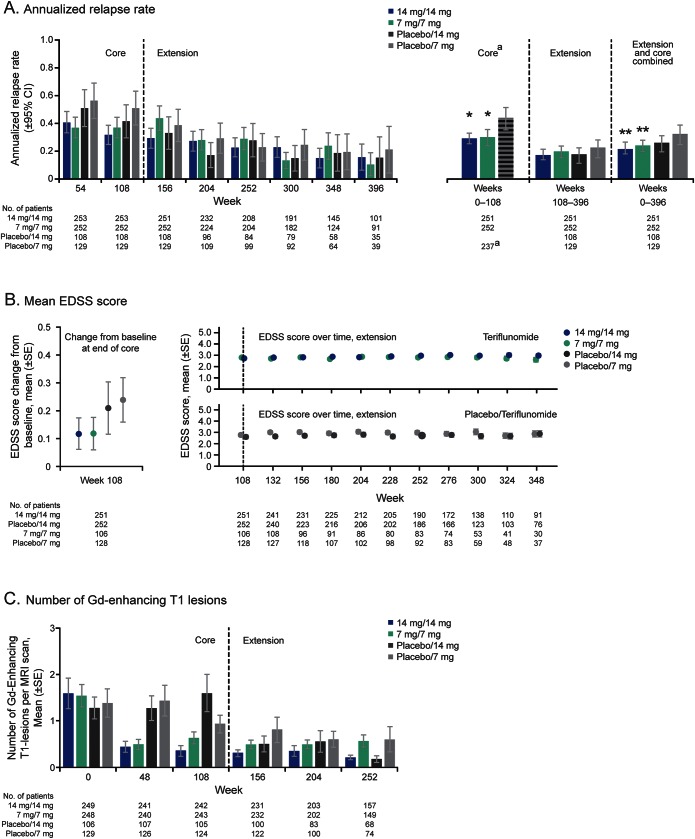
Figure 2. Efficacy outcomes in the core Teriflunomide Multiple Sclerosis Oral (TEMSO) and extension studies (modified intent-to-treat population).
(A) Annualized relapse rate. (B) Mean EDSS score. (C) Number of Gd-enhancing T1 lesions. a Patients receiving placebo in the core study are shown as one group for this analysis. Week refers to time since start of core study. Week 108 is end of core study/start of extension study. Total number of Gd-enhancing T1 lesions defined as all lesions enhanced on T1 scan. *p < 0.005 compared with pooled placebo group; **p < 0.05 compared with placebo/7-mg group. CI = confidence interval; EDSS = Expanded Disability Status Scale; Gd = gadolinium.