Abstract
Accessory scrotum is an unusual developmental anomaly defined as additional scrotal tissue in addition to a normally developed scrotum. The accessory scrotum arises posterior to the normally located scrotum and does not contain a testis. We report a case of an 18-month-old boy with an accessory scrotum attached to a perineal lipoma. We resected both and determined histologically that they were of the same tissue as the scrotum, including the presence of androgen receptor expression. To the best of our knowledge, this is the first case to assess androgen receptor expression in an accessory scrotum using immunostaining.
Keywords: Accessory scrotum, Perineal lipoma, Androgen receptor, Lipoblastoma
Introduction
Accessory scrotum is an unusual developmental anomaly with only 52 cases reported so far. Its pathogenesis remains unclear.1, 2 Here, we report a case of accessory scrotum attached to a perineal lipoma. We discuss the pathogenesis from the embryologic and histologic aspects of both accessory scrotum and perineal lipoma.
Case presentation
An 18-month-old boy was referred to our hospital for the treatment of perineal anomalies. The patient was born at 40 weeks of gestation, and his body weight was 3556 g. His parents were in good health, except for the stillbirth of a second male child related to meningocele, after the birth of the patient. The external appearance of the perineal anomalies had not changed over the patient's lifetime.
On physical examination at the first hospital visit, his penis was buried, and the scrotum appeared normal, containing 2 independent testes. At the right caudal area of the primary scrotum, a 30 × 30 × 20 mm, elastic, firm mass and a 20-mm long soft protruding portion with scrotum-like corrugations were visible from the apex of the mass. The median raphe was moderately recognizable and displaced to the left side, and his anus was normal (Fig. 1A). Extremity movement, defecation, and urination were all normal. No mass or dimple was observed in his dorsal area. There were no other developmental distortions except for the perineal area.
Figure 1.
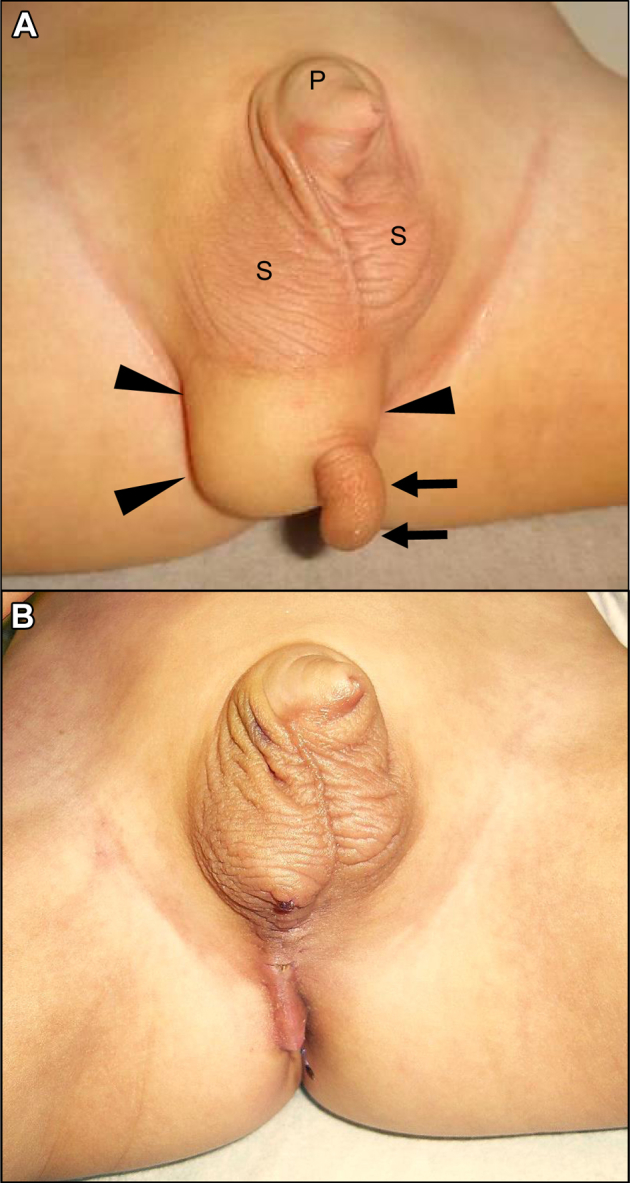
(A) Appearance of an accessory scrotum (arrow) attached to a perineal lipoma (arrowhead). The perineal lipoma is located in the right caudal area of the primary scrotum. (B) Appearance of the perineal area 1 month postoperatively. P, penis; S, scrotum.
On magnetic resonance imaging, the mass showed homogeneous high-intensity areas on both T1- and T2-weighted imaging, and its base reached just beneath the urethra and the anterior part of the rectum. The protruding portion displayed homogeneous low-intensity areas on both T1-and T2-weighted imaging. A voiding cystourethrogram revealed no abnormalities in the urethra. We determined the mass to be a tumor consisting of adipose tissue and the protruding portion to be a skin tumor.
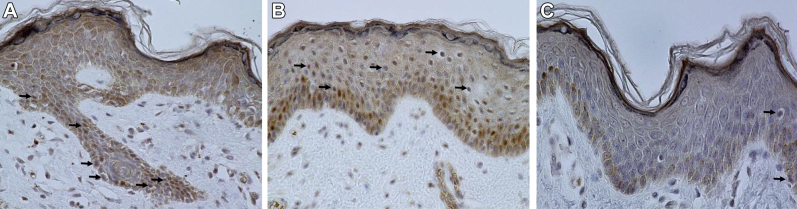
Under general anesthesia we resected the mass and protruding portion. We sutured the skin along the median line and formed the raphe as much as possible (Fig. 1B). Histologic examination revealed a perineal lipoma and scrotum-like skin (Fig. 2). We performed androgen receptor (AR) immunostaining as previously described3 and evaluated the density of AR-positive epidermal cells in the protruding portion (A), the primary scrotum (B), and the nonscrotal skin (C) to clarify the origin of the protruding portion. Monoclonal mouse antihuman AR antibody (clone AR441; Dako, Glostrup, Denmark) was used as primary antibody. AR-positive epidermal cells were observed in the protruding portion as well as in the normal scrotum at higher levels than in the nonscrotal skin (Fig. 3).
Figure 2.
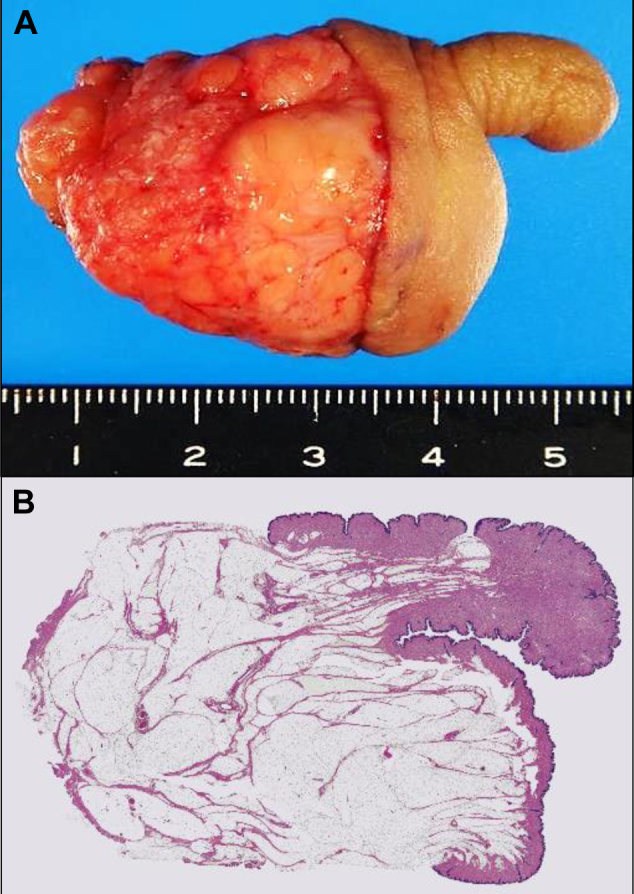
(A) Resected specimen of the accessory scrotum and perineal lipoma. (B) The mass is composed of adipose cells and was diagnosed as lipoma. The protruding portion is skin. Under the dermis, smooth muscle cells are evident, as in the primary scrotum (hematoxylin and eosin stain; original magnification ×1).
Figure 3.
Immunohistochemistry for androgen receptors (original magnification ×400). Androgen receptor–positive epidermal cells (arrow) are evident in the accessory scrotum (A) and primary scrotum (B) of this patient. In the nonscrotal skin (C), there are fewer androgen receptor–positive epidermal cells than in the primary or accessory scrotum.
The postoperative recovery was uneventful, and there was no recurrence at 9 months postoperatively.
Discussion
Accessory scrotum is defined as additional scrotal tissue in addition to a normally developed scrotum; however, an accessory scrotum does not contain testes and always arises posterior to the normally located scrotum.1 Histologic differences between scrotal and nonscrotal skin include smooth muscle cells under the scrotal dermis; there are no other differentiating characteristics. In this case, smooth muscle cells were evident in the protruding portion as well as in the normal scrotum.
From the embryology point of view, development of the external genitalia begins in the fifth week of gestation with the appearance of labioscrotal swellings lateral to the cloacal membrane.4 The labioscrotal swellings fuse and become the scrotum, and the midline forms the scrotal raphe.4 Dihydrotestosterone converted from testosterone by 5α-reductase is essential in the development of the scrotum during the early stages of gestation, and hemiscrotal agenesis may be the result of localized androgen insensitivity or 5α-reductase deficiency.3 If the external genitalia are not appropriately exposed to the androgen, they become female genitalia, a condition known as testicular feminization syndrome. In this case, AR-positive epidermal cells were observed in the protruding portion as well as in the normal scrotum at higher levels than in the nonscrotal skin (Fig. 3), indicating that the growth of the accessory scrotum was related to androgen exposure, similar to the primary scrotum.
Based on these findings of smooth muscle cells in the dermis and androgen receptor expression in the epidermal cells, we diagnosed the protruding portion as an accessory scrotum. To the best of our knowledge, this is the first report that has assessed androgen receptor expression in the accessory scrotum.
A literature search resulted in 52 cases of accessory scrotum, including our case. Thirty-seven cases (71%) were accompanied by perineal lipoma, 3 cases (6%) by perineal lipoblastoma, and only 12 cases (23%) were free of lipoma or lipoblastoma.1, 2 Given the high frequency of lipoma and lipoblastoma, these conditions are likely to be related to the pathogenesis of an accessory scrotum. Considering the displacement of the median raphe to one side in the present case, we hypothesized that aberrant adipose tissue in the perineum during the early phase of gestation disturbed the fusion of the labioscrotal folds, and, in turn, the remaining tissue became the accessory scrotum.
It is difficult to completely distinguish a lipoblastoma from a lipoma preoperatively, even by enhanced computed tomography or magnetic resonance imaging. Only 3 cases of accessory scrotum attached to a lipoblastoma have been reported so far, and of these, no recurrence has been reported after surgical resection.2 Conversely, of the lipoblastomas located elsewhere on the body, a recurrence rate of 46% has been reported, primarily related to incomplete resection.5 This suggests that an accessory scrotum attached to a lipoblastoma could also recur. Therefore, surgical excision of the accessory scrotum attached to adipose tissue is recommended not only for cosmetic reasons but also in the presence of a lipoblastoma.
Conclusion
In the present case, the histologic features, particularly the androgen receptor expression, of the accessory scrotum were similar to those of the primary scrotum. Accessory scrotum pathogenesis may be strongly related to the presence of a perineal lipoma or lipoblastoma. We recommend surgical excision of the accessory scrotum that is attached to adipose tissue.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. This case report was approved by the ethics committee of Nagoya City University Hospital.
Conflict of interest
None declared.
Acknowledgment
The 260th JUA Tokai Divisional Meeting Best Presentation Award was given for the summary of this case report.
Footnotes
Available online 5 October 2014
References
- 1.Sule J.D., Skoog S.J., Tank E.S. Perineal lipoma and the accessory labioscrotal fold: an etiological relationship. J Urol. 1994;151:475–477. doi: 10.1016/s0022-5347(17)34996-0. [DOI] [PubMed] [Google Scholar]
- 2.Kamal N.M., Jouini R., Yahya S., Haiba M. Benign intrascrotal lipoblastoma in a 4-month-old infant: a case report and review of literature. J Pediatr Surg. 2011;46:E9–E12. doi: 10.1016/j.jpedsurg.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Pichler R., Djedovic G., Klocker H. Quantitative measurement of the androgen receptor in prepuces of boys with and without hypospadias. BJU Int. 2013;112:265–270. doi: 10.1111/j.1464-410X.2012.11731.x. [DOI] [PubMed] [Google Scholar]
- 4.Flum A.S., Chaviano A.H., Kaplan W.E. Hemiscrotal agenesis: new variation in a rare anomaly. Urology. 2012;79:210–211. doi: 10.1016/j.urology.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Coffin C.M., Lowichik A., Putnam A. Lipoblastoma (LPB): a clinicopathologic and immunohistochemical analysis of 59 cases. Am J Surg Pathol. 2009;33:1705–1712. doi: 10.1097/PAS.0b013e3181b76462. [DOI] [PubMed] [Google Scholar]