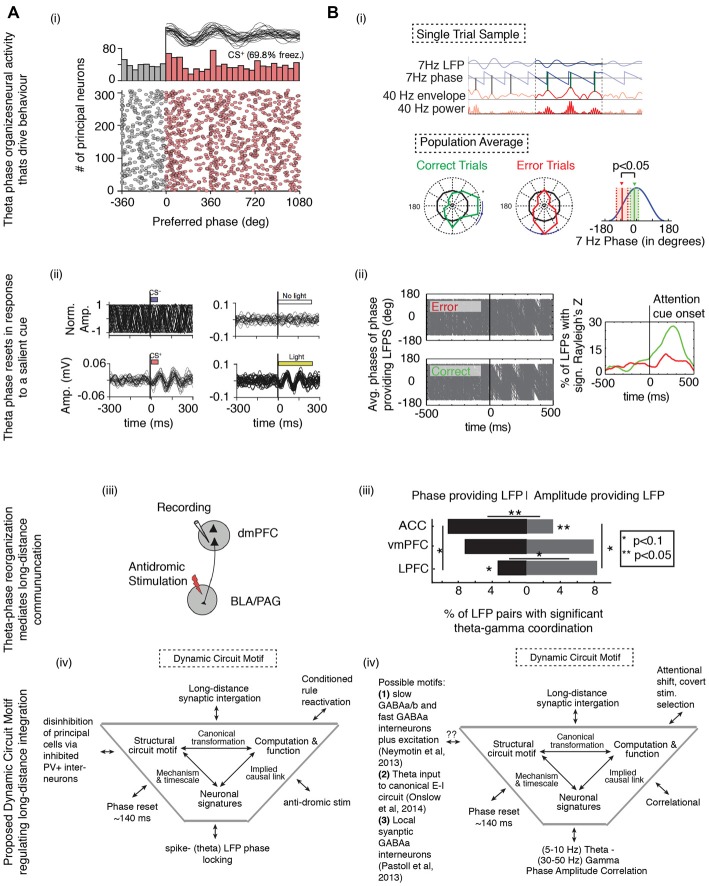
Figure 5.
Theta phase alignment to salient cues coordinates networks and correlates with behavioral success. Cue-aligned theta oscillations in the medial prefrontal cortices (i) organizes neural activity that drives behavior when (ii) the low frequency phases reset in response to a salient cue, and (iii) routes information across long-distances, all of which (iv) can be understood in terms of a Dynamic Circuit Motif, visualized near the bottom of the figure (for a review, see Womelsdorf et al., 2014b). (A) In a Pavlovian conditioning task, rodents learn to associate a neutral cue (conditioned stimulus, CS) with an aversive stimulus. Recordings were made in the rodent mPFC (adapted from Courtin et al., 2014). (i) Theta oscillations were often apparent when the animals exhibited freezing behavior. Neural spikes were locked to theta peaks, suggesting that when present, theta oscillations drove local circuit modulation that resulted in a fear response. (ii) Cue-aligned theta oscillations emerged in response to cue onset (left), but only when the animal was in a high (bottom) but not low (top) fear state. Optogenetically inducing theta oscillations also led to freezing (right). (iii) Neurons reorganized by theta projected to targets responsible for instantiating the fear response. Thus, theta phase reset in upstream areas is critical for affecting fear processing in downstream areas. (iv) A proposed dynamic circuit motif relating the reactivation of a conditioned rule (the function) to spike-theta phase locking (the neural signature). The long distance synaptic integration is achieved by inhibiting PV+ interneurons, leading to the disinhibition of projecting excitatory principal neurons. (B) In a cued selective attention task, macaques had to covertly shift the focus of attention to a target in order to correctly identify a change in the target (adapted from Voloh et al., 2015). (i) Following onset of the cue that triggered the attention shift, high frequency gamma amplitude was locked to the peaks of theta oscillations in prefrontal and anterior cingulate cortex. Importantly, gamma-amplitude to theta phase locking on correct trials was systemically different than on error trials. (ii) Correct, but not erroneous, covert attention shifts were also accompanied by an increase in theta phase consistency in those LFPs that modulated gamma activity. (iii) During theta-gamma correlation, LFPs with theta information predominated in the ACC, while those with gamma information were more likely in the LPFC. This study suggests that theta modulation of local activity may extend to distant sites. In line with the study in (A), theta phase reset drives neural reorganization, leading to observable behavioral changes. (iv) In the framework of a dynamic circuit motif, where a function (attention switching) is correlated with a neural signature (theta-gamma correlation). Causal connection between them could be subserved by neural elements known to generate gamma nested in theta oscillations. Abbreviations: BLA, basolateral amygdala; PGA, periaqueductal gray area; PV+, parvalbumin+.