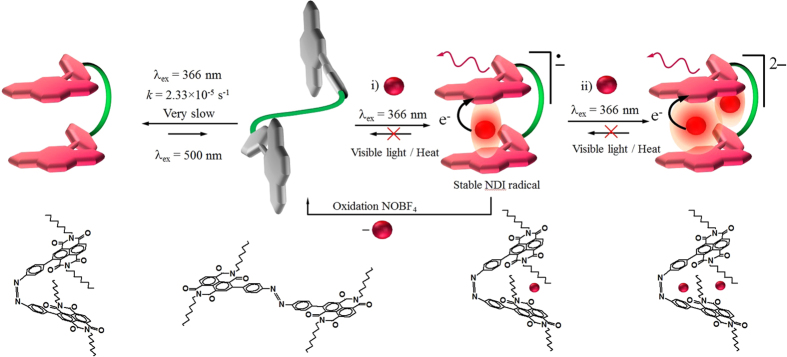
Figure 1.
Graphical representation: of the F− binding to the azo-NDI receptor in presence of UV light (366 nm) forming the cis radical anion in 1:1 fluoride to azo-NDI ratio in (i), and doubly charged complex with a 2:1 ratio in (ii). The electron transfer from F− to azo-NDI resulted in fluorochromogenic effect and also an enhancement of the emission output. UV irradiated trans-azo-NDI in the presence of fluoride ions converts it to the more stable cis isomer through NDI/F−/NDI sandwich complex. In the absence of fluoride under UV irradiation (366 nm) trans-azo-NDI converted to the cis conformation, however this exchange is very slow in the order of k = 2.33 × 10−5 S−1. Nevertheless, as expected cis-azo-NDI revert back to trans-form by irradiation with visible light (500 nm).