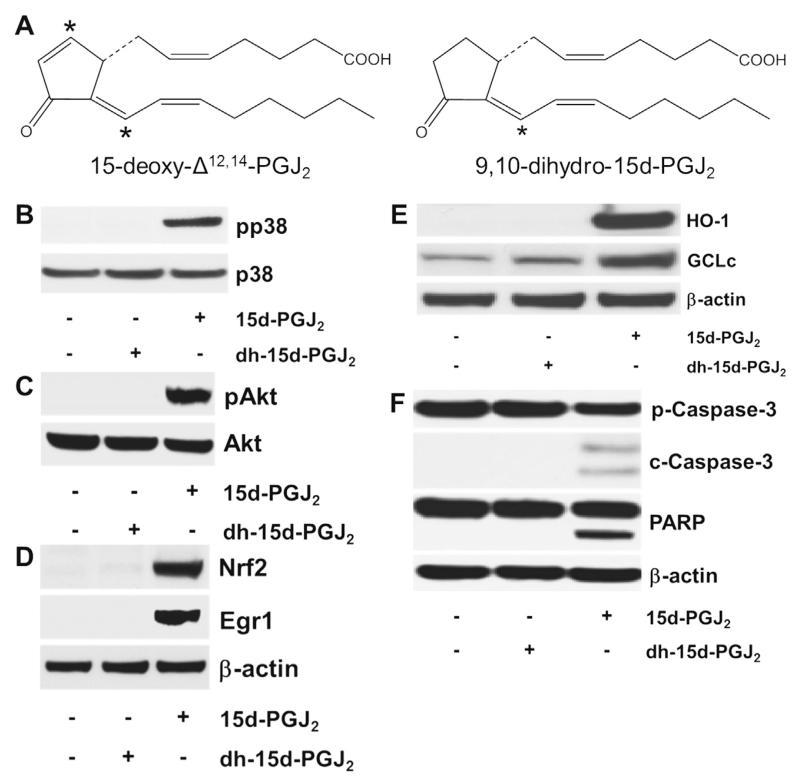
Fig. 10.
The chemical structure of (dh-)15d-PGJ2 and possible activation cascades. (A) The chemical structure of 15d-PGJ2 and dh-15d-PGJ2 (9,10-dihydro-15d-PGJ2, *indicates electrophilic carbon atom). (B–F) MG-63 cells were stimulated with dh-15d-PGJ2 (20 μM) or 15d-PGJ2 (20 μM, used as a positive control) for (B) 15 min to follow phosphorylation of p38 MAPK, (C) 1 h to follow phosphorylation of Akt, (D) 6 h to follow Nrf2 and Egr1 expression, (E) 6 h to follow HO-1 and GCLc expression or (F) 24 h to follow cleavage of pro-caspase and PARP. For Western blot analysis total protein lysates were subjected to SDS–PAGE. (B) Total p38 MAPK, (C) total Akt, and (D–F) β-actin were used as loading controls. One representative blot out of two is shown. Pro-caspase-3 (p-Caspase-3, molecular mass: 35 kDa); cleaved caspase-3 (c-Caspase-3; molecular mass: 19 and 17 kDa). Poly (ADP-ribose) polymerase (PARP; molecular mass: 116 kDa; cleaved PARP; molecular mass: 89 kDa). As membranes for Nrf2/Egr1 (D) were stripped and reprobed with anti-HO-1/-GCLc antibodies (E), the same β-actin blot is shown.