Abstract
Parkinson's disease (PD) is a common neurodegenerative disorder affecting patients in large numbers throughout the world. In this article, we review all the published data on PD based on studies in Indian population. We have tried to consolidate the contribution of Indian studies in PD research. We found 95 articles, of which 92 were original research papers. This is a relatively less number, but in the last decade, there has been an increase in research on PD from this country. But most of them seem to be restricted to only a few research institutes. The nonmotor symptoms and genetics are the most commonly studied aspects. The systematic review of the articles reveals that the epidemiology in India may be different with relatively lesser incidence here. Most of the genetic mutations found to cause PD in other population are not found in India, revealing that other genetic factors may be involved. Further research needs to be encouraged to understand the disease in Indian patients better, as all the results cannot be extrapolated from the Western literature to this heterogeneous Indian population. There need to be more studies on therapeutic aspects of the disease.
Keywords: Epidemiology, genetics, India, Parkinson's disease, treatment
Introduction
The first description of Parkinson's disease (PD) was given by James Parkinson in early 19th century.[1] But the knowledge about this disease has been present in India since ancient times. Though the prevalence of PD in India is less compared to other countries,[2] the total burden of PD is much higher as a result of large population. We reviewed all the published literature on PD from India till date. This will help in giving a comprehensive outlook regarding the previous and the current situation of this disease in our country. This review will also elaborate the contribution of India in the field of research in PD.
This systematic review of literature was undertaken according to the relevant criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We searched the PubMed database till December 31st, 2014 for studies in patients with PD that evaluated epidemiological characteristics pertaining to India. We applied a broad search strategy including the following terms: (Parkinson Disease AND India), (Parkinson Disease AND epidemiology), (Parkinson Disease AND India AND genetics), (Parkinson Disease AND India AND epidemiology), and (Parkinson Disease AND India AND treatment). The reference lists of included publications were also searched. Studies were eligible for inclusion if: i) PD was evaluated in India. The exclusion criteria were:
Parkinsonism apart from PD such as atypical Parkinsonism and secondary causes,
Animal studies,
Case reports, and
Pharmaceutical trials.
The search results have been given in Table 1. A total of 95 articles were according to the search criteria. The first published article from India on PD in indexed journals could be traced to 1988. In later years, the number of publications has steadily increased [Figure 1]. There are more publications in international journals (n = 76) than in national journals (n = 19). We attempted to categorize the articles on various aspects, such as clinical, epidemiology, genetics, pathogenesis, investigations, pathology, treatment, social issues, and physical rehabilitation. Of these, clinical aspects, nonmotor involvement (n = 33) and genetics (n = 25) were the most commonly studied categories [Table 2]. Most of the articles are published from only a few number of research institutes. Of the 95 articles, 86 were published from four institutes [Figure 2]. Further in this review, we elaborate the findings of the studies done on PD on patients from India.
Table 1.
PubMed search according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement

Figure 1.
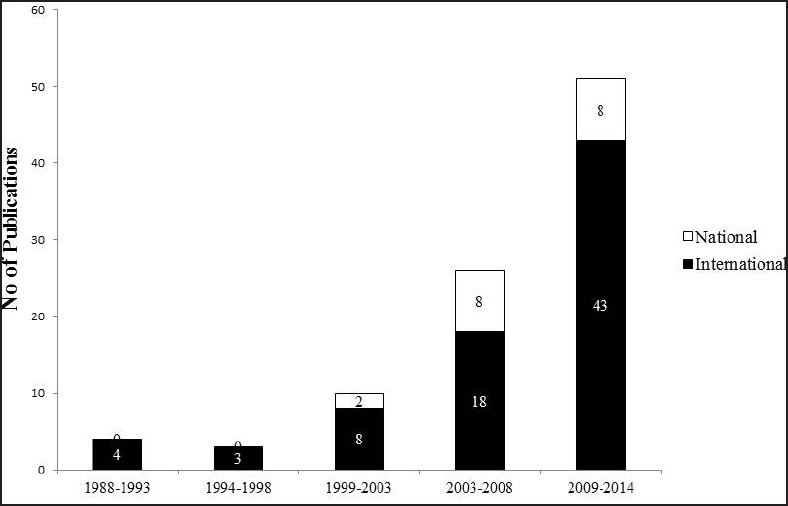
Number of publications on Parkinson's disease from India; year wise
Table 2.
Study categories of published articles on Parkinson's disease (PD) from India
Figure 2.
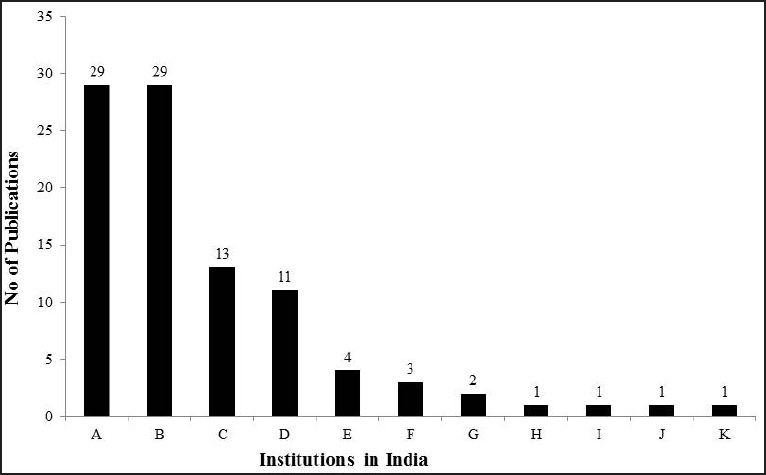
Number of publications on Parkinson's disease from India from various institutions. A = National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, B = All India Institute of Medical Sciences (AIIMS), New Delhi, C = Bangur Institute of Neurosciences/ Anthropology Society of India, Kolkata, D = NIMHANS+ AIIMS, E = Sree Chitra Tirunal Institute of Medical Sciences and Technology (SCT), Trivandrum, F = Madras Medical College (MMC), Chennai, G = Bombay Hospital, Mumbai, H = Neurospeciality Centre, Belgaum, I = Sher-e-Kashmir, Srinagar, J = Post Graduate Institute, Chandigarh, K = Parkinson's Disease Foundation, Bangalore + AIIMS
Epidemiology
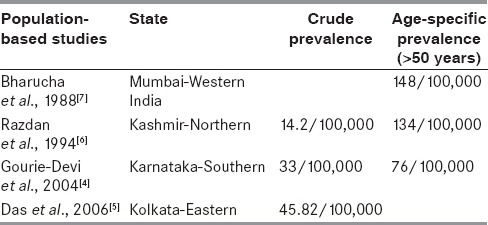
In a review by de Lau and Breteler in 2006, it was reported that an estimated 10 million people in the world (i.e., approximately 0.3% of the world population) and 1% of those above 60 years are found to be affected with PD.[3] There are very few population-based studies determining the exact incidence and prevalence of PD in India. In a door-to-door survey done in Bangalore district in South Karnataka in India in 2004, the prevalence rate of Parkinsonism was found to be 33 per 100,000 (crude prevalence) and 76 per 100,000 (age adjusted).[4] Rural population had a higher prevalence compared to the urban population (41 vs 14). But it was less compared to other highly prevalent neurological disorders such as headache, epilepsy, stroke, and mental retardation. From a survey in Kolkata in 2006, the prevalence of Parkinsonism was found to be 45.82 per 100,000.[5] In the state of Kashmir,[6] the prevalence was 14.1 per 100,000, while the age adjusted prevalence was 134 per 100,000. A survey, done in Parsi community in Mumbai, a small stable community,[7] showed a prevalence of 192 per 100,000, which was higher compared to rest of the population. In a surveillance in old age homes in a Bangalore,[8] there was very high prevalence of 17.6% (109/612 residents) of Parkinsonism. This may be due to unawareness of this disorder among the general population who do not avail the medical facilities at the appropriate time.
The prevalence rate in Europe is 1.8 per 100 in population above 65 years.[9] As the prevalence of PD in non-white population is lower, the normal human brains from India and United Kingdom were compared, to determine the cause.[10] The neurons which were melanized in substantia nigra (pars compacta), as the age increases, were counted and compared. Indian brains, though had low number of melanized neurons, the decrease in these number of neurons with age was much less compared to the whites. But a similar study comparing Nigerian and British brains[11] did not show any difference between these two ethnic groups. In one clinical study comparing the prevalence of PD in different ethnic groups in India,[12] Anglo-Indians, who had admixed ethnicity with Indian population had nearly five times lesser prevalence of PD compared to general Indian population. In summary, there may be several other factors causing the difference in prevalence in varied populations and this need to be explored.
In most studies, it has been found that women have lesser prevalence of PD.[3] A study at All India Institute of Medical Sciences (AIIMS) by Yadav et al.,[13] showed that women with higher number and longer duration of pregnancies had lesser prevalence of PD. This may indicate a protective role of estrogen.
Most of the prevalence studies have used different methods to screen the population. Tanner et al.,[14] devised one of the widely used validated screening questionnaires for PD. Sarangmath et al., from National Institute of Mental Health and Neurosciences (NIMHANS)[15] have modified the screening questionnaire by Tanner et al., to cater to the varying literacy levels and different languages used in India. A few changes were made from the prior standard screening questionnaire and this was found to have good sensitivity even when administered by a nonmedical assistant.
The epidemiology of PD in India is different compared to populations from different countries and also among different ethnicities in India [Table 3]. The prevalence studies in India are done among small population from specific areas probably with a particular type of cultural background. So, to generalize it to the rest of the population in a country where there is a huge cultural, ethnic, and social difference may not be correct. Hence, large multicenter studies for evaluating prevalence at different parts of the country are necessary.
Table 3.
Epidemiology of Parkinson's disease (PD) in India
Risk Factors
There are several risk factors and protective factors which have been described in development of PD. In a study on Indian population by Behari et al.,[16] the risk factors included that of male gender who had higher incidence (1:3.96) similar to that reported in other countries.[17,18,19] However, this data may be confounded by the fact that in India, women compared to men, are less likely to seek or have access to medical attention. Family history of PD and previous history of depression were the other risk factors likely to predispose to PD. Environmental risk factors such as rural living, well water drinking, farming, and pesticide exposure have been described previously.[20] The only significant environmental risk factor seemed to be the well water drinking in the study from India. Tobacco smoking and exposure to pets had a protective effect from PD. The protection from tobacco smoking in this study was found to be much higher compared to previous studies,[21,22,23] with five times decreased risk. It has been speculated by the author that bidi smoking (different form of tobacco consumed in India), may have a higher protection due to presence of higher levels of carbon monoxide, tar, nicotine, ammonia, and volatile phenols.[24]
Another demographic and clinical profile study of 557 PD patients in northern Karnataka district by Kadakola et al., showed that male gender (64.8%) was more affected. In this study, the prevalence was not higher in rural community as in other previous studies.[25]
Clinical features
Till recently, PD was considered to be a geriatric disease. However, in the past few decades it has been well established that several genetic and familial forms of PD can occur. Early onset PD (EOPD) with onset of motor symptoms at £40 years, constitute approximately 5-10% of patients.[26] EOPD can be further divided into young onset PD (YOPD; age at onset 20-40 years) and juvenile PD (JP; age of onset <20 years). In an Indian study comparing the clinical features of EOPD,[27] patients with JP had a higher incidence of dystonia, family history of Parkinsonism, and abnormalities in autonomic function tests [Table 4].
Table 4.
Studies on motor and nonmotor symptoms of Parkinson's disease (PD) from India
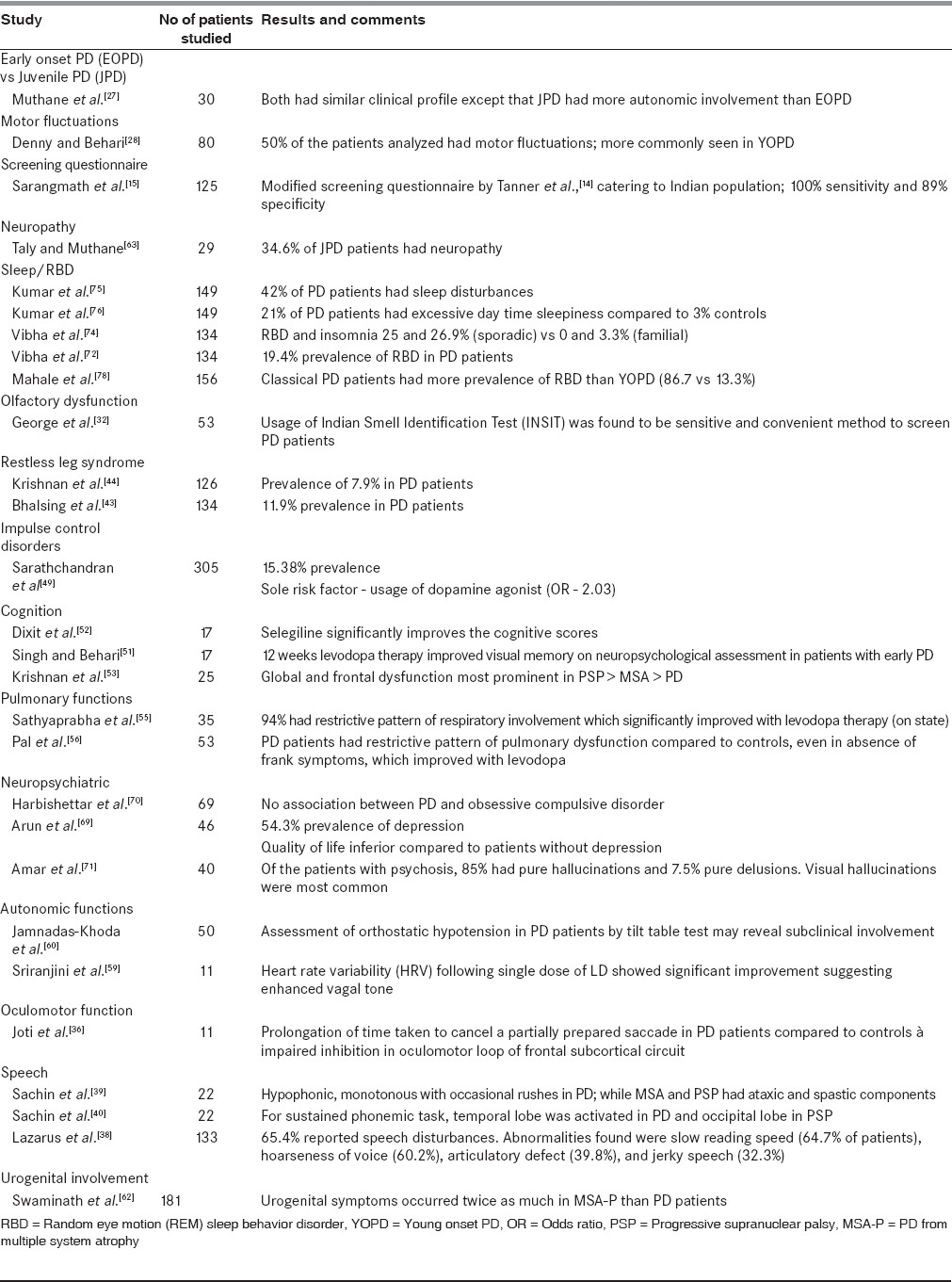
Motor symptoms
An unselected PD population attending AIIMS was studied by Denny and Behari to assess the factors responsible for motor fluctuations.[28] Similar to a previous study,[29] the prevalence of dyskinesias was around 50% in Indian population, though the mean dose of LD causing dyskinesias was much lower.
Nonmotor symptoms
PD has a large spectrum of clinical features ranging from motor to nonmotor symptoms. Some of these nonmotor symptoms can result in greater morbidity than the motor symptoms themselves. Some of the nonmotor symptoms can occur as part of aging. PD patients and age-matched healthy controls were assessed by Krishnan et al.,[30] using Nonmotor Symptom Scale questionnaire,[31] to determine if there is any difference in the occurrence of these symptoms and the burden caused by them. The prevalence of these symptoms was higher and more severe in PD patients compared to normal ageing people. Cardiovascular symptoms, perceptual problems, hallucinations, cognition, and mood changes were significantly more in PD patients.
Olfactory dysfunction can precede motor symptoms in PD Indian Smell identification Test (INSIT),[32] and was designed and compared with the standard Sniffin’ Stick test. The smells used in this test were those that are familiar in the Indian population. INSIT showed a sensitivity of 79.2% and specificity of 78%. INSIT has been recommended as cheaper and convenient alternative to test for olfactory function in the Indian population.
Basal ganglia is known to be involved in response inhibition through frontal subcortical circuits.[33] Studies using no-no go test have shown the involvement of somatomotor loop of frontal subcortical circuit in PD patients.[34,35] An Indian study by Joti et al.,[36] assessed the oculomotor loop by testing a visually-guided saccadic task. Compared to normal subjects, PD patients were found to have delayed switching of the saccade from one target to another. This proves that basal ganglia are not only involved in the somatomotor loop but also in the oculomotor loop of frontal subcortical circuit.
Speech impairment has been reported in 60-80% of the PD patients which reaches up to 100% in the later stages.[37] Lazarus et al., subjected PD patients to the Indian Speech and Hearing Association (ISHA) articulation assessment and the Vaghmi software.[38] It was found that 64.7% had slow reading speed, 60.2% hoarseness of voice, 39.8% articulatory defect, and 32.3% jerky speech. Articulation defects were most commonly seen with the labial syllables (42.1%), followed by the lingual and the palatal.
In a study by Sachin et al., the speech profiles of PD patients were compared with patients with other types of atypical Parkinsonism such as progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). PD patients had hypophonic monotonous speech with occasional rushes of speech, while PSP and MSA had spastic and ataxic components.[39] Sachin et al., further evaluated the central mechanisms for speech impairment in PD patients using functional magnetic resonance imaging (fMRI) studies. They compared these results with PSP patients.[40] It was found that for a sustained phonemic task in fMRI, superior temporal gyrus was activated in PD and occipital cortex in PSP.
Restless leg syndrome (RLS) is known to occur in PD patients with its prevalence ranging from 0.5 to 19.5%.[41,42] Bhalsing et al., at NIMHANS compared the prevalence of RLS in PD and atypical parkinsonism. Its prevalence was found to be higher (11.9%) in PD compared to other parkinsonian neurodegenerative disorders and controls.[43] Only one patient each in MSA and PSP and none in diffuse Lewy body dementia (DLB) were diagnosed to have RLS. The clinical features, apart from the sleep disturbances, did not vary between those patients with or without RLS. A previous study at AIIMS by Krishnan et al., has reported a prevalence of 7.9% in PD patients.[44]
Impulse control disorders (ICD) are common in patients undergoing dopaminergic therapy. The prevalence of ICD is reported to vary in studies done in different countries, ranging from 3.5 to 35%.[45,46,47,48] Sarathchandran et al., studied its occurrence in Indian PD population and reported a prevalence of 31.6%.[49] These patients were having at least one ICD, punding being the most common (15.7%). Few of these patients (7.5%) had more than one ICD. Treatment of PD patients with dopamine agonist was found to be the most important factor for the occurrence of ICD in them.
Memory disturbances and dementia are known to occur in later stages of PD. Patients with early PD can have subtle disturbances in neuropsychological testing.[50] To evaluate whether these abnormalities were affected by treatment, PD patients were assessed after 12 weeks of levodopa (LD) therapy.[51] Singh and Behari found that there was deterioration in visual memory after LD therapy, though the verbal memory was not affected. On the contrary, Dixit et al., found that, selegiline improved the memory function, cognition, and prevented the prolongation of P300 latency, which is a marker of cognitive function.[52] In a study from Sree Chitra Tirunal Institute of Medical Sciences and Technology (SCTIMST), comparing the patients with PD and the patients with atypical parkinsonism, the cognitive function was most affected in PSP followed by MSA and least affected in PD.[53]
Respiratory dysfunction in PD is well-known and causes both obstructive and restrictive patterns.[54] In a study conducted in India by Sathyaprabha et al.,[55] the effect on pulmonary functions in asymptomatic PD patients after treatment with LD was analyzed. Of these, 94% had restrictive pattern, signifying that there may be chest wall rigidity which contributed to the low chest wall compliance. The dysfunction was mitigated with medications (LD), probably due to the reduction in rigidity and bradykinesia leading to better coordination of muscles. In another similar study by Pal et al.,[56] women were found to be more affected, with poorer pulmonary functions. The pulmonary functions improved with the administration of LD, though they did not improve up to the control values. Routine pulmonary function monitoring, even in asymptomatic patients, may be necessary to anticipate any significant respiratory dysfunction and consequent effective intervention.
Autonomic dysfunction is well-known in PD and is found to be present in 76% of the patients.[57] The cardiac autonomic dysfunction manifests as decreased response of heart rate and blood pressure to autonomic stimulation.[58] There are only a few studies from India evaluating autonomic functions in PD. Sriranjini et al., evaluated the effect of single dose of LD on cardiac autonomic function in PD. It was found that LD improves the heart rate variability due to increased vagal tone.[59]
Orthostatic hypotension (OH) has been reported in 20-58% of PD. But only a few patients may report orthostatic symptoms. Jamnadas-Khoda et al., interviewed 50 PD patients to verify if orthostatic symptoms reported by patients provide evidence of underlying orthostatic hypotension. It was found that only 18% of the patients who had OH during tilt table test reported symptoms prior to tilting. Orthostatic symptoms have a high specificity but low sensitivity for detection of OH in patients with PD. For subclinical detection of OH, a screening tilt test with prolonged tilting (>3 min) is recommended for all PD patients, as the blood pressure (BP) fall may be delayed in these patients.[60] Raghothaman et al., studied patients of PD and PD from MSA (MSA-P) to find if the presence of dysautonomic symptoms such as urinary incontinence and orthostatic symptoms, would help in the diagnosis of these disorders. It was found that when these symptoms are present within 1 year of illness, they can accurately point towards the diagnosis of MSA-P.[61,62]
Genetically mediated PD is associated with other unusual manifestations such as peripheral neuropathy. Juvenile PD patients from India, when evaluated for subclinical involvement using electroneuromyography, more than 50% had abnormalities.[63] They had chronic partial denervation with reinnervation (34.6%), abnormalities of motor conduction (13.8%) and sensory conduction (31.9%), and abnormal sympathetic skin response (37.9%).
Several neuropsychiatric manifestations such as depression, anxiety, and sleep disturbances have been described in PD. The prevalence of depression in PD patients ranges from 7.7 to 76%,[64,65] of which community-based studies[64] showed lower prevalence. Depression in PD may be reactive depression as a result of chronic illness as well as a result of neurodegeneration. The degeneration of subcortical nuclei such as hypothalamus, locus coeruleus, dorsal raphe, and ventral tegmental area and loss of dopamine and noradrenaline innervation in the limbic system have been implicated as a cause of depression in the PD patients.[66,67] Depression affects the cognition and quality of life (QOL).[68] A study comparing PD and other chronic illnesses[69] revealed higher prevalence of depression in PD. They also had significantly higher disability and poorer QOL. As frontobasal circuitry is involved in obsessive compulsive disorder (OCD), some studies have tried to find if there is any association between PD and OCD. Harbishettar et al., in a study of 69 PD patients with normal cognition and 69 matched medically-ill controls, failed to show any association between OCD and PD.[70] This could be due to the reason that different circuitries may be involved in these two disorders.
Psychosis can also be a part of nonmotor spectrum of PD. It may be disabling to the patients and its presence may also warrant change in treatment strategies. The manifestations include hallucinations and delusions. In a study from India by Amar et al., 40 patients of PD with psychosis were assessed. Pure hallucinations were commonest in these patients (85%) and a combination of delusions and hallucinations was found in 7.5%.[71] Of these visual hallucinations were predominant, amounting to 60%.
Random eye motion (REM) sleep behavior disorder (RBD) can precede PD motor symptoms by many years. In an evaluation of RBD in PD patients in India,[72] the prevalence was 19.2%, similar to that found from other studies.[73] Comparing the clinical features of patients with or without RBD, visual hallucinations and sleep disturbances such as insomnia and snoring were more in the RBD group. Comparing the sleep disturbances in idiopathic and familial forms of PD,[74] RBD and insomnia was higher in idiopathic PD. Other sleep disorders apart from RBD which have been reported from Indian population[75] include insomnia (32%), nightmares (32%), and excessive day time sleepiness (15%). Excessive day time sleepiness correlated with higher Unified Parkinson's Disease Rating Scale (UPDRS) and Hoehn and Yahr stage.[76] Patients with sleep disturbances are more susceptible for mood disorders, have decreased QOL and increased caregiver burden.[77] Comparing the young and late onset PD,[78] Mahale et al., found that RBD was more common in older patients and those who were in advanced stage of the disease. Those with RBD had poorer quality of sleep and excessive daytime sleepiness.
Postural imbalance forms one of the important features of PD. Till date there are only few studies that addressed the quantitative evaluation of balance impairment in PD. Ganesan et al., used dynamic post urography to evaluate 20 PD patients with no symptoms of postural imbalance and normal pull test.[79] There was direction-specific imbalance, predominantly in forward-right and backward-left positions in PD patients compared to the controls. Based on these findings, the authors suggested that a modified diagonal pull test may be helpful to detect subtle postural imbalance in early stage of PD. Nallegowda et al., in their pilot study showed that the imbalance in PD is due to a combined effect of reduction in muscle strength, decreased proprioception, visual sense, and narrow base of support.[80] In on state, muscle strength, gait speed, and use of ankle strategy improve significantly.
Genetics
Genetic PD, found in less than 10% of PD, is caused by single gene Mendelian disorder. The idiopathic type of PD is attributed to the complex interaction between genetic susceptibility and environmental factors.[26] There is a significant difference in prevalence of PD in India compared to other population, and there are differences in the normal brain specimens of different ethnic populations.[10,11] So it is likely that the genetic factors causing PD may also vary in different populations. So it was hypothesized that the incidence and profile of genetic forms of PD in Indian population may be different.[81] Several studies have reported that the genetic forms of PD in India may differ from those occurring in the Western countries [Table 5].
Table 5.
Genetics of Parkinson's disease (PD) in India
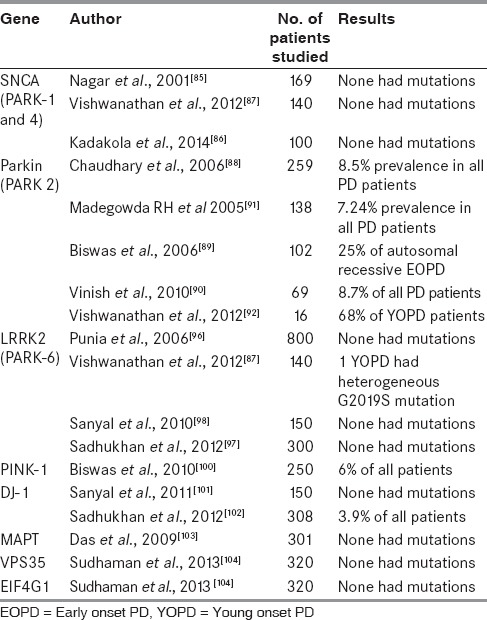
Alpha-synuclein (SNCA) gene mutations causing PARK-1 and PARK-4, is found in 1.7-2.7%[82,83] of patients with familial PD and 0.3% in patients with isolated PD[84] in different populations. In the three Indian studies from northern and southern regions, none of the patients had mutations of SNCA gene.[85,86,87] Parkin gene mutations which are autosomal recessive, are the commonest mutations in YOPD. Prevalence of parkin gene in PD has been reported from five studies in India. In these studies,[88,89,90] the presence of parkin gene in all patients of PD was found to be similar ranging from 7.24 to 8.7%. However, when parkin mutations were specifically looked for in EOPD,[91] Parkin mutations were present in 2% of all EOPD, 10% of familial EOPD, and 25% of autosomal recessive EOPD. Only one study showed very high prevalence of about 68% in YOPD patients.[92] Studies from other countries have reported the incidence of parkin in YOPD which ranged from 3.1 to 49%.[93,94] So, the Indian population seem to have relatively lesser incidence of parkin mutation.
Leucine-rich repeat kinase 2 (LRRK2) gene mutations cause autosomal dominant late onset forms of PD. Prevalence of these mutations have been found to be 5.1%.[95] In India, none of the patients from four tertiary centers studies had these mutations.[87,96,97,98] Phosphatase and tensin (PTEN) homolog induced kinase-1 (PINK-1) gene mutations are responsible for PARK-6, comprising 9.3% of patients with EOPD.[99] In India, the screening for this gene mutation in EOPD found a prevalence of 7.2%.[100] While a study showed no association of DJ-1 mutations causing PARK 7 in eastern Indian patients,[101] another study found 3.9% of PD patients from the same region having these mutations.[102] Evaluation of microtubule associated protein tau mutations (MAPT),[103] a neuronal protein, in patients with PD in eastern India revealed no significant association with PD. Screening for vacuolar protein sorting homolog 35 (VPS35) and eukaryotic translation initiation factor 4 gamma 1 (EIF4G1) genes, causing PARK17 and PARK 18, in Indian population also turned out to be negative.[104] VPS35 mutations have been found to constitute 0.5-1.67% of PD[105,106] in various ethnic groups, while EIF4G1 mutations were found in 7.37% of Caucasians with PD.[107]
Other genetic studies which have been done on Indian PD population include those on polymorphisms of N-acetyl transferase 2 gene,[108] dopamine receptor and transporter gene,[109] and dopamine synthesis and metabolism genes.[110] One study evaluated the role of single nucleotide polymorphisms (SNPs) in drug-metabolizing enzymes (DMEs) and the oxidative stress pathway and found that there was association of these SNPs with YOPD.[111] Deoxyribonucleic acid (DNA) stability was analyzed from autopsied brains of Indian patients with PD and was found that there was increased fragmentation in basal ganglia and hippocampus.[112]
In summary, there are only very few studies from India that have systematically looked into the genetic profile of PD patients in India. Though the data from the available studies indicate that the underlying genetic factors in causation of PD may be different from the other populations, there needs to be more research in this area before any conclusions can be drawn.
Imaging
There are only a few clinical studies on imaging in PD reported from India. Gupta et al., compared the pattern of mineralization in susceptibility weighted imaging (SWI) in PD and atypical Parkinsonism.[113] The hypointensity score of red nucleus, putamen, and substantia nigra was found to be significantly more in patients with PSP compared to PD and MSA.
Electrophysiology and Transcranial Magnetic Stimulation (TMS)
Visual evoked potentials (VEP) in PD patients have been known to be abnormal with prolonged P100 seen in two-thirds of the patients observed.[114,115] Muthane et al., studied abnormalities in evoked potentials in EOPD patients. Evoked potentials, both visual and auditory, were assessed in EOPD and found to be abnormal in 26% each. There was higher incidence of brainstem auditory evoked potential (BAEP) abnormalities in EOPD compared to classical PD.[116]
Transmagnetic stimulation is an important tool used to assess the central motor circuitry and has been used extensively to study the pathophysiology of PD. In India, there have been a few studies of TMS on PD patients at SCTIMST and AIIMS. Bhatia et al., studied about threshold intensity (TI) in PD patients.[117] Studies from SCTIMST elaborated about long-term potentiation and depression (LTP and LTD) in PD,[118] its effect after acute dopamine therapy,[119] and also the effect of cerebellar inhibition on motor plasticity in patients with LD-induced dyskinesias (LIDs).[120]
Neurochemistry and Pathology
There are a few studies from India assessing the effect of oxidative enzymes, and antioxidants in the pathogenesis of PD. Abraham et al., from AIIMS studied 115 PD patients and measured the antioxidant enzymes in these patients. It was found that superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (G-Px), and glucose-6-phosphate dehydrogenase (G6PD) were low in PD patients compared to the controls.[121] Sanyal et al., studied plasma malondialdehyde levels, a marker of lipid peroxidation in 80 PD patients and found them to be higher than in controls.[122] In a study done on autopsied brains of PD patients at NIMHANS, it was found that there was increased glutathione (GSH) and astrocyte proliferation in non-substantia nigra regions, which may have a protective role in these areas of the brain.[123]
A study by Muthane et al., at NIMHANS compared the normal human brains from India and from United Kingdom.[10] The neurons which were melanized in substantia nigra (pars compacta), as the age increases, were counted and compared. Indian brains, though had low number of melanized neurons, the decrease in these number of neurons with age was much less compared to the white population. This may give us a clue regarding the relatively lesser incidence of PD in Indian population.
Treatment
Studies on therapeutic aspects of PD from India are scarce. Denny and Behari from AIIMS studied about motor fluctuations in PD and found that 50% were affected with LID. A lower mean LD dose of more than 337.5 mg/day was associated with the presence of LID.[28]
There are a few centers in India which offer surgical treatment for PD and consequently there are limited studies on surgical treatment of PD from India. There was evaluation of long-term effects of subthalamic nucleus (STN) stimulation in 45 PD patients carried out over 5 years by Kishore et al.[124] There was a sustained improvement of the cardinal signs of the disease, the QOL, and the motor complications; while axial symptoms, emotional, and social aspects did not have such sustained improvement. Naskar et al., studied the effect of deep brain stimulation (DBS) on long latency event-related potentials, which showed an increased N100 latency, reflecting the worsening of orientation response with STN stimulation, but there was no change in P300.[125]
In a series of 29 patients who underwent pallidotomy for PD, Kishore et al., explored the functional somatotopy of globuspallidus (GPi).[126] They found that ventral GPi lesion significantly improved LID.
In India, many patients seek alternative medicine, especially in chronic diseases. Ayurveda is the most common alternative medicine available in the country. For PD, their treatment includes a mixture of cow's milk, Mucuna pruriens, Hyoscyamus reticulatus seeds, Withania somnifera, and Sida cordifolia roots.[127] In this study, UPDRS and activities of daily living were assessed following the administration of above medications. Those who underwent cleansing or eliminating therapy (panchakarma) prior to the intake of medicines had significantly better outcomes.
Physical therapy, which is noninvasive, is an essential part of treatment for PD. Srikumar et al., from AIIMS studied 28 patients of PD undergoing pharmacological therapy. They found that a systematic program of exercises improves the UPDRS scores, activities of daily living, and gait.[128] As freezing and gait disturbances are difficult symptoms to treat, both medically and surgically, new strategies of physical therapies, like partial weight supported treadmill gait training (PWSTT)[129] and wireless vibratory feedback system called PD shoe[130] have been found to be useful. PWSTT was also found to influence baroreflex sensitivity (BRS) showing significant improvement within 4 weeks of training by Ganesan et al., from NIMHANS.[131]
In India, a patient spends around 16-41.7% of the income for medications.[132] Though cost of treatment in India is lesser, it is still out of reach of many patients. To consider surgical options, when necessary, may be much more difficult in patients of lower socioeconomic status.
QOL
Patients with PD, because of their disability, can have poor QOL—physical, psychological, and social. Several studies have shown significant decrease in QOL in these patients.[133,134] The important clinical variables found to cause lower QOL in India were depression, disease severity, disability in off periods, dyskinesias, postural instability, gait disturbances, and cognitive dysfunction. The QOL was also found to be influenced by disease stage, severity, duration, and financial security.[135] Indians had lesser disturbances in family and communal relationships due to the disease, probably due to stronger family bonds in their families. In this study, only 25% of patients responded to questions regarding sexual functions. This was probably due to the inhibitions among Indian people to discuss the sexual issues.
To find if the patients and caregivers had enough knowledge about the disorder, an assessment was done using a questionnaire by Yadav et al., at AIIMS.[136] They were found to be well-informed regarding most of the clinical and treatment aspects of the disease. The information they were lacking was regarding surgery and biochemical abnormalities. But it is not known if the patients attending primary care centers would have similar knowledge. The factors associated with increased caregiver burden in Indian patients are depression, high UPDRS, and sleep disturbances. However, multiple caregivers decreased the burden.[77]
Conclusions
This review shows that there is paucity of literature about PD in India. There are very few centers which are doing research in this field. This review shows that the Indian population may differ from the rest of the world in the context of PD, be it epidemiology or genetics or response to treatment. But systematic large-scale studies are scarce to make definite conclusions. The social and psychological issues in our patients are also different. More research needs to be encouraged, so that the characteristics of the disease in the Indian population can be understood better. We would suggest that elaborate research on genetic and therapeutic aspects is necessary. A combined effort of neurologists and other clinicians like psychiatrists, physical therapists, and basic sciences personnel would help in improving the quality of research. This would lead to better treatment options tailored to the needs of our patients in India.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
References
- 1.Pearce JM. Aspects of the history of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1989;Suppl:6–10. doi: 10.1136/jnnp.52.suppl.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singhal B, Lalkaka J, Sankhla C. Epidemiology and treatment of Parkinson's disease in India. Parkinsonism Relat Disord. 2003;9(Suppl 2):S105–9. doi: 10.1016/s1353-8020(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 3.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 4.Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna DK. Prevalence of neurological disorders in Bangalore, India: A community-based study with a comparison between urban and rural areas. Neuroepidemiology. 2004;23:261–8. doi: 10.1159/000080090. [DOI] [PubMed] [Google Scholar]
- 5.Das SK, Biswas A, Roy T, Banerjee TK, Mukherjee CS, Raut DK, et al. A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124:163–72. [PubMed] [Google Scholar]
- 6.Razdan S, Kaul RL, Motta A, Kaul S, Bhatt RK. Prevalence and pattern of major neurological disorders in rural Kashmir (India) in 1986. Neuroepidemiology. 1994;13:113–9. doi: 10.1159/000110368. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of Parkinson's disease in the Parsi community of Bombay, India. Arch Neurol. 1988;45:1321–3. doi: 10.1001/archneur.1988.00520360039008. [DOI] [PubMed] [Google Scholar]
- 8.Ragothaman M, Murgod UA, Gururaj G, Louis ED, Subbakrishna DK, Muthane UB. High occurrence and low recognition of Parkinsonism (and possible PD) in old age homes in Bangalore, South India. J Assoc Physicians India. 2008;56:233–6. [PubMed] [Google Scholar]
- 9.de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21–3. [PubMed] [Google Scholar]
- 10.Muthane U, Yasha TC, Shankar SK. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann Neurol. 1998;43:283–7. doi: 10.1002/ana.410430304. [DOI] [PubMed] [Google Scholar]
- 11.Muthane UB, Chickabasaviah YT, Henderson J, Kingsbury AE, Kilford L, Shankar SK, et al. Melanized nigral neuronal numbers in Nigerian and British individuals. Mov Disord. 2006;21:1239–41. doi: 10.1002/mds.20917. [DOI] [PubMed] [Google Scholar]
- 12.Ragothaman M, Murgod UA, Gururaj G, Kumaraswamy SD, Muthane U. Lower risk of Parkinson's disease in an admixed population of European and Indian origins. Mov Disord. 2003;18:912–4. doi: 10.1002/mds.10449. [DOI] [PubMed] [Google Scholar]
- 13.Yadav R, Shukla G, Goyal V, Singh S, Behari M. A case control study of women with Parkinson's disease and their fertility characteristics. J Neurol Sci. 2012;319:135–8. doi: 10.1016/j.jns.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 14.CM T, DW G, CG G. A brief screening questionnaire for parkinsonism [abstract] Ann Neurol. 1990:267–88. [Google Scholar]
- 15.Sarangmath N, Rattihalli R, Ragothaman M, Gopalkrishna G, Doddaballapur S, Louis ED, et al. Validity of a modified Parkinson's disease screening questionnaire in India: Effects of literacy of participants and medical training of screeners and implications for screening efforts in developing countries. Mov Disord. 2005;20:1550–6. doi: 10.1002/mds.20576. [DOI] [PubMed] [Google Scholar]
- 16.Behari M, Srivastava AK, Das RR, Pandey RM. Risk factors of Parkinson's disease in Indian patients. J Neurol Sci. 2001;190:49–55. doi: 10.1016/s0022-510x(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 17.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology. 2004;63:1240–4. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 18.Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, Porta-Etessam J, Trincado R, Vega S, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62:734–41. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 19.Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, et al. Parkinson's disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55:1358–63. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- 20.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: A metaanalysis. Environ Res. 2001;86:122–7. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 21.Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: A case-control study in Germany. Neurology. 1996;46:1275–84. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- 22.De Michele G, Filla A, Volpe G, De Marco V, Gogliettino A, Ambrosio G, et al. Environmental and genetic risk factors in Parkinson's disease: A case-control study in southern Italy. Mov Disord. 1996;11:17–23. doi: 10.1002/mds.870110105. [DOI] [PubMed] [Google Scholar]
- 23.Hofman A, Collette HJ, Bartelds AI. Incidence and risk factors of Parkinson's disease in The Netherlands. Neuroepidemiology. 1989;8:296–9. doi: 10.1159/000110197. [DOI] [PubMed] [Google Scholar]
- 24.Rahman M, Fukui T. Bidi smoking and health. Public Health. 2000;114:123–7. doi: 10.1038/sj.ph.1900625. [DOI] [PubMed] [Google Scholar]
- 25.Kadakol G, Kulkarni SS, Kulkarni BB, Kulkarni SS, Bhaskar LVKS, Wali GM, Nadgir D, Hiremath SV, Gai PB. Parkinson's disease in North Karnataka: An epidemiological perspective. J Anthropol. 2012;8 1 – ISSN 1973- 2880. [Google Scholar]
- 26.Lesage S, Brice A. Parkinson's disease: From monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 27.Muthane UB, Swamy HS, Satishchandra P, Subhash MN, Rao S, Subbakrishna D. Early onset Parkinson's disease: Are juvenile- and young-onset different? Mov Disord. 1994;9:539–44. doi: 10.1002/mds.870090506. [DOI] [PubMed] [Google Scholar]
- 28.Denny AP, Behari M. Motor fluctuations in Parkinson's disease. J Neurol Sci. 1999;165:18–23. doi: 10.1016/s0022-510x(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 29.Markham CH, Diamond SG. Evidence to support early levodopa therapy in Parkinson disease. Neurology. 1981;31:125–31. doi: 10.1212/wnl.31.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan S, Sarma G, Sarma S, Kishore A. Do nonmotor symptoms in Parkinson's disease differ from normal aging? Mov Disord. 2011;26:2110–3. doi: 10.1002/mds.23826. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: The NMSQuest study. Mov Disord. 2006;21:916–23. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 32.George J, Jose T, Behari M. Use of Indian smell identification test for evaluating olfaction in idiopathic Parkinson's disease patients in India. Neurol India. 2013;61:365–70. doi: 10.4103/0028-3886.117598. [DOI] [PubMed] [Google Scholar]
- 33.Strick PL, Dum RP, Mushiake H. Basal ganglia ‘loops’ with the cerebral cortex. Functions of the cortico-basal ganglia loop. 1995:106–124. [Google Scholar]
- 34.Ilinsky IA, Jouandet ML, Goldman-Rakic PS. Organization of the nigrothalamocortical system in the rhesus monkey. J Comp Neurol. 1985;236:315–30. doi: 10.1002/cne.902360304. [DOI] [PubMed] [Google Scholar]
- 35.Cooper JA, Sagar HJ, Tidswell P, Jordan N. Slowed central processing in simple and go/no-go reaction time tasks in Parkinson's disease. Brain. 1994;117:517–29. doi: 10.1093/brain/117.3.517. [DOI] [PubMed] [Google Scholar]
- 36.Joti P, Kulashekhar S, Behari M, Murthy A. Impaired inhibitory oculomotor control in patients with Parkinson's disease. Exp Brain Res. 2007;177:447–57. doi: 10.1007/s00221-006-0687-0. [DOI] [PubMed] [Google Scholar]
- 37.Streifler M, Hofman S. Disorders of verbal expression in parkinsonism. Adv Neurol. 1984;40:385–93. [PubMed] [Google Scholar]
- 38.Lazarus JP, Vibha D, Handa KK, Singh S, Goyal V, Srivastava T, et al. A study of voice profiles and acoustic signs in patients with Parkinson's disease in North India. J Clin Neurosci. 2012;19:1125–9. doi: 10.1016/j.jocn.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Sachin S, Shukla G, Goyal V, Singh S, Aggarwal V, Gureshkumar, et al. Clinical speech impairment in Parkinson's disease, progressive supranuclear palsy, and multiple system atrophy. Neurol India. 2008;56:122–6. doi: 10.4103/0028-3886.41987. [DOI] [PubMed] [Google Scholar]
- 40.Sachin S, Senthil Kumaran S, Singh S, Goyal V, Shukla G, Mahajan H, et al. Functional mapping in PD and PSP for sustained phonation and phoneme tasks. J Neurol Sci. 2008;273:51–6. doi: 10.1016/j.jns.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Angelini M, Negrotti A, Marchesi E, Bonavina G, Calzetti S. A study of the prevalence of restless legs syndrome in previously untreated Parkinson's disease patients: Absence of co-morbid association. J Neurol Sci. 2011;310:268–8. doi: 10.1016/j.jns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Nomura T, Inoue Y, Miyake M, Yasui K, Nakashima K. Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson's disease. Mov Disord. 2006;21:380–4. doi: 10.1002/mds.20734. [DOI] [PubMed] [Google Scholar]
- 43.Bhalsing K, Suresh K, Muthane UB, Pal PK. Prevalence and profile of Restless Legs Syndrome in Parkinson's disease and other neurodegenerative disorders: A case-control study. Parkinsonism Relat Disord. 2013;19:426–30. doi: 10.1016/j.parkreldis.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson's disease: A case-controlled study. Mov Disord. 2003;18:181–5. doi: 10.1002/mds.10307. [DOI] [PubMed] [Google Scholar]
- 45.Fan W, Ding H, Ma J, Chan P. Impulse control disorders in Parkinson's disease in a Chinese population. Neurosci Lett. 2009;465:6–9. doi: 10.1016/j.neulet.2009.06.074. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY, Kim JM, Kim JW, Cho J, Lee WY, Kim HJ, et al. Association between the dose of dopaminergic medications and the behavioral disturbances in Parkinson disease. Parkinsonism Relat Disord. 2010;16:202–7. doi: 10.1016/j.parkreldis.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Auyeung M, Tsoi TH, Tang WK, Cheung CM, Lee CN, Li R, et al. Impulse control disorders in Chinese Parkinson's disease patients: The effect of ergot derived dopamine agonist. Parkinsonism Relat Disord. 2011;17:635–7. doi: 10.1016/j.parkreldis.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Lim SY, Tan ZK, Ngam PI, Lor TL, Mohamed H, Schee JP, et al. Impulsive-compulsive behaviours are common in Asian Parkinson's disease patients: Assessment using the QUIP. Parkinsonism Relat Disord. 2011;17:761–4. doi: 10.1016/j.parkreldis.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Sarathchandran P, Soman S, Sarma G, Krishnan S, Kishore A. Impulse control disorders and related behaviors in Indian patients with Parkinson's disease. Mov Disord. 2013;28:1901–2. doi: 10.1002/mds.25557. [DOI] [PubMed] [Google Scholar]
- 50.Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain. 1991;114(Pt 5):2095–122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Behari M. Verbal and visual memory in patients with early Parkinson's disease: Effect of levodopa. Neurol India. 2006;54:33–7. doi: 10.4103/0028-3886.24699. [DOI] [PubMed] [Google Scholar]
- 52.Dixit SN, Behari M, Ahuja GK. Effect of selegiline on cognitive functions in Parkinson's disease. J Assoc Physicians India. 1999;47:784–6. [PubMed] [Google Scholar]
- 53.Krishnan S, Mathuranath PS, Sarma S, Kishore A. Neuropsychological functions in progressive supranuclear palsy, multiple system atrophy and Parkinson's disease. Neurol India. 2006;54:268–72. doi: 10.4103/0028-3886.27150. [DOI] [PubMed] [Google Scholar]
- 54.Sabate M, Rodriguez M, Mendez E, Enriquez E, Gonzalez I. Obstructive and restrictive pulmonary dysfunction increases disability in Parkinson disease. Arch Phys Med Rehabil. 1996;77:29–34. doi: 10.1016/s0003-9993(96)90216-6. [DOI] [PubMed] [Google Scholar]
- 55.Sathyaprabha TN, Kapavarapu PK, Pall PK, Thennarasu K, Raju TR. Pulmonary functions in Parkinson's disease. Indian J Chest Dis Allied Sci. 2005;47:251–7. [PubMed] [Google Scholar]
- 56.Pal PK, Sathyaprabha TN, Tuhina P, Thennarasu K. Pattern of subclinical pulmonary dysfunctions in Parkinson's disease and the effect of levodopa. Mov Disord. 2007;22:420–4. doi: 10.1002/mds.21330. [DOI] [PubMed] [Google Scholar]
- 57.Magalhaes M, Wenning GK, Daniel SE, Quinn NP. Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson's disease--a retrospective comparison. Acta Neurol Scand. 1995;91:98–102. doi: 10.1111/j.1600-0404.1995.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 58.Mesec A, Sega S, Trost M, Pogacnik T. The deterioration of cardiovascular reflexes in Parkinson's disease. Acta Neurol Scand. 1999;100:296–9. [PubMed] [Google Scholar]
- 59.Sriranjini SJ, Ganesan M, Datta K, Pal PK, Sathyaprabha TN. Effect of a single dose of standard levodopa on cardiac autonomic function in Parkinson's disease. Neurol India. 2011;59:659–63. doi: 10.4103/0028-3886.86536. [DOI] [PubMed] [Google Scholar]
- 60.Jamnadas-Khoda J, Koshy S, Mathias CJ, Muthane UB, Ragothaman M, Dodaballapur SK. Are current recommendations to diagnose orthostatic hypotension in Parkinson's disease satisfactory? Mov Disord. 2009;24:1747–51. doi: 10.1002/mds.22537. [DOI] [PubMed] [Google Scholar]
- 61.Ragothaman M, Swaminath PV, Sarangmath N, Koshy S, Adhyam M, Subbakrishna DK, et al. Role of dysautonomic symptoms in distinguishing Parkinson's disease (PD) from multiple system atrophy (MSA-P) within a year of developing motor symptoms. J Assoc Physicians India. 2011;59:95–8. [PubMed] [Google Scholar]
- 62.Swaminath PV, Ragothaman M, Koshy S, Sarangmath N, Adhyam M, Subbakrishna DK, et al. Urogenital symptoms in Parkinson's disease and multiple system atrophy-Parkinsonism: At onset and later. J Assoc Physicians India. 2010;58:86–90. [PubMed] [Google Scholar]
- 63.Taly AB, Muthane UB. Involvement of peripheral nervous system in juvenile Parkinson's disease. Acta Neurol Scand. 1992;85:272–5. doi: 10.1111/j.1600-0404.1992.tb04043.x. [DOI] [PubMed] [Google Scholar]
- 64.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease. A community-based study. Arch Neurol. 1996;53:175–9. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- 65.Happe S, Schrodl B, Faltl M, Muller C, Auff E, Zeitlhofer J. Sleep disorders and depression in patients with Parkinson's disease. Acta Neurol Scand. 2001;104:275–80. doi: 10.1034/j.1600-0404.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- 66.Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 67.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: Loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(Pt 6):1314–22. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 68.Schrag A. Quality of life and depression in Parkinson's disease. J Neurol Sci. 2006;248(1-2):151–7. doi: 10.1016/j.jns.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 69.Arun MP, Bharath S, Pal PK, Singh G. Relationship of depression, disability, and quality of life in Parkinson's disease: A hospital-based case-control study. Neurol India. 2011;59:185–9. doi: 10.4103/0028-3886.79133. [DOI] [PubMed] [Google Scholar]
- 70.Harbishettar V, Pal PK, Janardhan Reddy YC, Thennarasu K. Is there a relationship between Parkinson's disease and obsessive-compulsive disorder? Parkinsonism Relat Disord. 2005;11:85–8. doi: 10.1016/j.parkreldis.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Amar BR, Yadav R, Janardhan Reddy YC, Pal PK. A clinical profile of patients with Parkinson's disease and psychosis. Ann Indian Acad Neurol. 2014;17:187–92. doi: 10.4103/0972-2327.132625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vibha D, Shukla G, Goyal V, Singh S, Srivastava AK, Behari M. RBD in Parkinson's disease: A clinical case control study from North India. Clin Neurol Neurosurg. 2011;113:472–6. doi: 10.1016/j.clineuro.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008;79:387–91. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 74.Vibha D, Shukla G, Singh S, Goyal V, Srivastava AK, Behari M. Lower prevalence of sleep disturbances in familial versus sporadic Parkinson's disease: A questionnaire based study. J Neurol Sci. 2010;295:27–30. doi: 10.1016/j.jns.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 75.Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson's disease. Mov Disord. 2002;17:775–81. doi: 10.1002/mds.10167. [DOI] [PubMed] [Google Scholar]
- 76.Kumar S, Bhatia M, Behari M. Excessive daytime sleepiness in Parkinson's disease as assessed by Epworth Sleepiness Scale (ESS) Sleep Med. 2003;4:339–42. doi: 10.1016/s1389-9457(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 77.Agrawal V, Goyal V, Shukla G, Behari M. Predictors of caregivers’ burden of Parkinson's disease in India: Experience of a tertiary care center in India. J Parkinsons Restless Legs Syndrome. 2012;2:59–65. [Google Scholar]
- 78.Mahale R, Yadav R, Pal PK. Rapid eye movement sleep behaviour disorder in young- and older-onset Parkinson disease: A questionnaire-based study. Sleep Med. 2014;15:642–6. doi: 10.1016/j.sleep.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Ganesan M, Pal PK, Gupta A, Sathyaprabha TN. Dynamic posturography in evaluation of balance in patients of Parkinson's disease with normal pull test: Concept of a diagonal pull test. Parkinsonism Relat Disord. 2010;16:595–9. doi: 10.1016/j.parkreldis.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Nallegowda M, Singh U, Handa G, Khanna M, Wadhwa S, Yadav SL, et al. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's disease: A pilot study. Am J Phys Med Rehabil. 2004;83:898–908. doi: 10.1097/01.phm.0000146505.18244.43. [DOI] [PubMed] [Google Scholar]
- 81.Muthane U, Jain S, Gururaj G. Hunting genes in Parkinson's disease from the roots. Med Hypotheses. 2001;57:51–5. doi: 10.1054/mehy.2001.1289. [DOI] [PubMed] [Google Scholar]
- 82.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–9. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 83.Ibanez P, Lesage S, Janin S, Lohmann E, Durif F, Destee A, et al. Alpha-synuclein gene rearrangements in dominantly inherited parkinsonism: Frequency, phenotype, and mechanisms. Arch Neurol. 2009;66:102–8. doi: 10.1001/archneurol.2008.555. [DOI] [PubMed] [Google Scholar]
- 84.Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, et al. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–9. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 85.Nagar S, Juyal RC, Chaudhary S, Behari M, Gupta M, Rao SN, et al. Mutations in the alpha-synuclein gene in Parkinson's disease among Indians. Acta Neurol Scand. 2001;103:120–2. doi: 10.1034/j.1600-0404.2001.103002120.x. [DOI] [PubMed] [Google Scholar]
- 86.Kadakol GS, Kulkarni SS, Wali GM, Gai PB. Molecular analysis of alpha-synuclein gene in Parkinson's disease in North Karnataka, India. Neurol India. 2014;62:149–52. doi: 10.4103/0028-3886.132338. [DOI] [PubMed] [Google Scholar]
- 87.Vishwanathan Padmaja M, Jayaraman M, Srinivasan AV, Srikumari Srisailapathy CR, Ramesh A. The SNCA (A53T, A30P, E46K) and LRRK2 (G2019S) mutations are rare cause of Parkinson's disease in South Indian patients. Parkinsonism Relat Disord. 2012;18:801–2. doi: 10.1016/j.parkreldis.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Chaudhary S, Behari M, Dihana M, Swaminath PV, Govindappa ST, Jayaram S, et al. Parkin mutations in familial and sporadic Parkinson's disease among Indians. Parkinsonism Relat Disord. 2006;12:239–45. doi: 10.1016/j.parkreldis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Biswas A, Gupta A, Naiya T, Das G, Neogi R, Datta S, et al. Molecular pathogenesis of Parkinson's disease: Identification of mutations in the Parkin gene in Indian patients. Parkinsonism Relat Disord. 2006;12:420–6. doi: 10.1016/j.parkreldis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Vinish M, Prabhakar S, Khullar M, Verma I, Anand A. Genetic screening reveals high frequency of PARK2 mutations and reduced Parkin expression conferring risk for Parkinsonism in North West India. J Neurol Neurosurg Psychiatry. 2010;81:166–70. doi: 10.1136/jnnp.2008.157255. [DOI] [PubMed] [Google Scholar]
- 91.Madegowda RH, Kishore A, Anand A. Mutational screening of the parkin gene among South Indians with early onset Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76:1588–90. doi: 10.1136/jnnp.2004.046888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padmaja MV, Jayaraman M, Srinivasan AV, Srisailapathy CR, Ramesh A. PARK2 gene mutations in early onset Parkinson's disease patients of South India. Neurosci Lett. 2012;523:145–7. doi: 10.1016/j.neulet.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 93.Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, et al. French Parkinson's Disease Genetics Study Group; European Consortium on Genetic Susceptibility in Parkinson's Disease. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–7. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 94.Haylett WL, Keyser RJ, du Plessis MC, van der Merwe C, Blanckenberg J, Lombard D, et al. Mutations in the parkin gene are a minor cause of Parkinson's disease in the South African population. Parkinsonism Relat Disord. 2012;18:89–92. doi: 10.1016/j.parkreldis.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 95.Khan NL, Jain S, Lynch JM, Pavese N, Abou-Sleiman P, Holton JL, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: Clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128(Pt 12):2786–96. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 96.Punia S, Behari M, Govindappa ST, Swaminath PV, Jayaram S, Goyal V, et al. Absence/rarity of commonly reported LRRK2 mutations in Indian Parkinson's disease patients. Neurosci Lett. 2006;409:83–8. doi: 10.1016/j.neulet.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 97.Sadhukhan T, Vishal M, Das G, Sharma A, Mukhopadhyay A, Das SK, et al. Evaluation of the role of LRRK2 gene in Parkinson's disease in an East Indian cohort. Dis Markers. 2012;32:355–62. doi: 10.3233/DMA-2012-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanyal J, Sarkar B, Ojha S, Banerjee TK, Ray BC, Rao VR. Absence of commonly reported leucine-rich repeat kinase 2 mutations in Eastern Indian Parkinson's disease patients. Genet Test Mol Biomarkers. 2010;14:691–4. doi: 10.1089/gtmb.2010.0054. [DOI] [PubMed] [Google Scholar]
- 99.Rogaeva E, Johnson J, Lang AE, Gulick C, Gwinn-Hardy K, Kawarai T, et al. Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease. Arch Neurol. 2004;61:1898–904. doi: 10.1001/archneur.61.12.1898. [DOI] [PubMed] [Google Scholar]
- 100.Biswas A, Sadhukhan T, Majumder S, Misra AK, Das SK, Variation Consortium IG, et al. Evaluation of PINK1 variants in Indian Parkinson's disease patients. Parkinsonism Relat Disord. 2010;16:167–71. doi: 10.1016/j.parkreldis.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Sanyal J, Sarkar B, Banerjee TK, Mukherjee SC, Ray BC, Raghavendra Rao V. Evaluating intra-genetic variants of DJ-1 among Parkinson's disease patients of eastern India. Neurol Res. 2011;33:349–53. doi: 10.1179/016164110X12767786356679. [DOI] [PubMed] [Google Scholar]
- 102.Sadhukhan T, Biswas A, Das SK, Ray K, Ray J. DJ-1 variants in Indian Parkinson's disease patients. Dis Markers. 2012;33:127–35. doi: 10.3233/DMA-2012-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das G, Misra AK, Das SK, Ray K, Ray J. Microtubule-associated protein tau (MAPT) influences the risk of Parkinson's disease among Indians. Neurosci Lett. 2009;460:16–20. doi: 10.1016/j.neulet.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 104.Sudhaman S, Behari M, Govindappa ST, Muthane UB, Juyal RC, Thelma BK. VPS35 and EIF4G1 mutations are rare in Parkinson's disease among Indians. Neurobiol Aging. 2013;34:2442.e1–3. doi: 10.1016/j.neurobiolaging.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 105.Ando M, Funayama M, Li Y, Kashihara K, Murakami Y, Ishizu N, et al. VPS35 mutation in Japanese patients with typical Parkinson's disease. Mov Disord. 2012;27:1413–7. doi: 10.1002/mds.25145. [DOI] [PubMed] [Google Scholar]
- 106.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–7. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chartier-Harlin MC, Dachsel JC, Vilarino-Guell C, Lincoln SJ, Lepretre F, Hulihan MM, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89:398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chaudhary S, Behari M, Dihana M, Swaminath PV, Govindappa ST, Jayaram S, et al. Association of N-acetyl transferase 2 gene polymorphism and slow acetylator phenotype with young onset and late onset Parkinson's disease among Indians. Pharmacogenet Genomics. 2005;15:731–5. doi: 10.1097/01.fpc.0000173485.59430.49. [DOI] [PubMed] [Google Scholar]
- 109.Juyal RC, Das M, Punia S, Behari M, Nainwal G, Singh S, et al. Genetic susceptibility to Parkinson's disease among South and North Indians: I. Role of polymorphisms in dopamine receptor and transporter genes and association of DRD4 120-bp duplication marker. Neurogenetics. 2006;7:223–9. doi: 10.1007/s10048-006-0048-y. [DOI] [PubMed] [Google Scholar]
- 110.Punia S, Das M, Behari M, Mishra BK, Sahani AK, Govindappa ST, et al. Role of polymorphisms in dopamine synthesis and metabolism genes and association of DBH haplotypes with Parkinson's disease among North Indians. Pharmacogenet Genomics. 2010;20:435–41. doi: 10.1097/FPC.0b013e32833ad3bb. [DOI] [PubMed] [Google Scholar]
- 111.Punia S, Das M, Behari M, Dihana M, Govindappa ST, Muthane UB, et al. Leads from xenobiotic metabolism genes for Parkinson's disease among north Indians. Pharmacogenet Genomics. 2011;21:790–7. doi: 10.1097/FPC.0b013e32834bcd74. [DOI] [PubMed] [Google Scholar]
- 112.Hegde ML, Gupta VB, Anitha M, Harikrishna T, Shankar SK, Muthane U, et al. Studies on genomic DNA topology and stability in brain regions of Parkinson's disease. Arch Biochem Biophys. 2006;449:143–56. doi: 10.1016/j.abb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 113.Gupta D, Saini J, Kesavadas C, Sarma PS, Kishore A. Utility of susceptibility-weighted MRI in differentiating Parkinson's disease and atypical parkinsonism. Neuroradiology. 2010;52:1087–94. doi: 10.1007/s00234-010-0677-6. [DOI] [PubMed] [Google Scholar]
- 114.Bodis-Wollner I, Yahr MD. Measurements of visual evoked potentials in Parkinson's disease. Brain. 1978;101:661–71. doi: 10.1093/brain/101.4.661. [DOI] [PubMed] [Google Scholar]
- 115.Bodis-Wollner I, Onofjr M. The visual system in Parkinson's disease. Adv Neurol. 1987;45:323–7. [PubMed] [Google Scholar]
- 116.Muthane UB, Satishchandra P, Subhash MN. Visual and auditory evoked potentials in early onset Parkinson's disease and their relationship to cerebrospinal fluid monoamine metabolites. Mov Disord. 1993;8:344–8. doi: 10.1002/mds.870080316. [DOI] [PubMed] [Google Scholar]
- 117.Bhatia M, Johri S, Behari M. Increased cortical excitability with longer duration of Parkinson's disease as evaluated by transcranial magnetic stimulation. Neurol India. 2003;51:13–5. [PubMed] [Google Scholar]
- 118.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–5. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- 119.Kishore A, Popa T, Velayudhan B, Joseph T, Balachandran A, Meunier S. Acute dopamine boost has a negative effect on plasticity of the primary motor cortex in advanced Parkinson's disease. Brain. 2012;135(Pt 7):2074–88. doi: 10.1093/brain/aws124. [DOI] [PubMed] [Google Scholar]
- 120.Kishore A, Popa T, Balachandran A, Chandran S, Pradeep S, Backer F, et al. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson's disease: Clues from dyskinetic patients. Cereb Cortex. 2014;24:2055–67. doi: 10.1093/cercor/bht058. [DOI] [PubMed] [Google Scholar]
- 121.Abraham S, Soundararajan CC, Vivekanandhan S, Behari M. Erythrocyte antioxidant enzymes in Parkinson's disease. Indian J Med Res. 2005;121:111–5. [PubMed] [Google Scholar]
- 122.Sanyal J, Bandyopadhyay SK, Banerjee TK, Mukherjee SC, Chakraborty DP, Ray BC, et al. Plasma levels of lipid peroxides in patients with Parkinson's disease. Eur Rev Med Pharmacol Sci. 2009;13:129–32. [PubMed] [Google Scholar]
- 123.Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, et al. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson's disease brains. Neurochem Res. 2011;36:1452–63. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- 124.Kishore A, Rao R, Krishnan S, Panikar D, Sarma G, Sivasanakaran MP, et al. Long-term stability of effects of subthalamic stimulation in Parkinson's disease: Indian Experience. Mov Disord. 2010;25:2438–44. doi: 10.1002/mds.23269. [DOI] [PubMed] [Google Scholar]
- 125.Naskar S, Sood SK, Goyal V. Effect of acute deep brain stimulation of the subthalamic nucleus on auditory event-related potentials in Parkinson's disease. Parkinsonism Relat Disord. 2010;16:256–60. doi: 10.1016/j.parkreldis.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 126.Kishore A, Panikar D, Balakrishnan S, Joseph S, Sarma S. Evidence of functional somatotopy in GPi from results of pallidotomy. Brain. 2000;123(Pt 12):2491–500. doi: 10.1093/brain/123.12.2491. [DOI] [PubMed] [Google Scholar]
- 127.Nagashayana N, Sankarankutty P, Nampoothiri MR, Mohan PK, Mohanakumar KP. Association of L-DOPA with recovery following Ayurveda medication in Parkinson's disease. J Neurol Sci. 2000;176:124–7. doi: 10.1016/s0022-510x(00)00329-4. [DOI] [PubMed] [Google Scholar]
- 128.Srikumar V, Wadhwa S, Singh U, Yadav SL, Behari M, Dwivedi SN. Structured Rehabilitation Exercise Program in Parkinson's Disease. Ind J Phys Med and Rehabil. 2010;21:31–36. [Google Scholar]
- 129.Ganesan M, Sathyaprabha TN, Gupta A, Pal PK. Effect of partial weight-supported treadmill gait training on balance in patients with Parkinson disease. PMR. 2014;6:22–33. doi: 10.1016/j.pmrj.2013.08.604. [DOI] [PubMed] [Google Scholar]
- 130.Winfree KN, Pretzer-Aboff I, Hilgart D, Aggarwal R, Behari M, Agrawal SK. The effect of step-synchronized vibration on patients with Parkinson's disease: Case studies on subjects with freezing of gait or an implanted deep brain stimulator. IEEE Trans Neural Syst Rehabil Eng. 2013;21:806–11. doi: 10.1109/TNSRE.2013.2250308. [DOI] [PubMed] [Google Scholar]
- 131.Ganesan M, Pal PK, Gupta A, Sathyaprabha TN. Treadmill gait training improves baroreflex sensitivity in Parkinson's disease. Clin Auton Res. 2014;24:111–8. doi: 10.1007/s10286-014-0236-z. [DOI] [PubMed] [Google Scholar]
- 132.Ragothaman M, Govindappa ST, Rattihalli R, Subbakrishna DK, Muthane UB. Direct costs of managing Parkinson's disease in India: Concerns in a developing country. Mov Disord. 2006;21:1755–8. doi: 10.1002/mds.21035. [DOI] [PubMed] [Google Scholar]
- 133.Kuopio AM, Marttila RJ, Helenius H, Toivonen M, Rinne UK. The quality of life in Parkinson's disease. Mov Disord. 2000;15:216–23. doi: 10.1002/1531-8257(200003)15:2<216::aid-mds1003>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 134.Karlsen KH, Tandberg E, Arsland D, Larsen JP. Health related quality of life in Parkinson's disease: A prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69:584–9. doi: 10.1136/jnnp.69.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ray J, Das SK, Gangopadhya PK, Roy T. Quality of life in Parkinson's disease — Indian scenario. J Assoc Physicians India. 2006;54:17–21. [PubMed] [Google Scholar]
- 136.Yadav R, Shukla G, Goyal V, Singh S, Behari M. Knowledge of Parkinson's disease among patients and caregivers attending movement disorder clinic at a tertiary care centre in north India. Ann Indian Acad Neurol. 2012;15:294–6. doi: 10.4103/0972-2327.104339. [DOI] [PMC free article] [PubMed] [Google Scholar]


