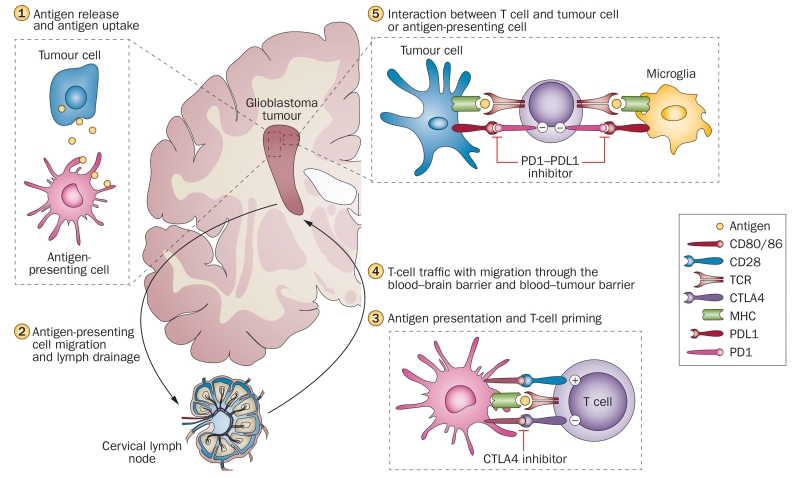
Figure 1.
Overview of the immune response and major immune checkpoint molecules in the immune cycle of glioblastoma. Antigens released from degenerating tumour cells are taken up by antigen-presenting cells, microglia and macrophages (1). Antigens are trafficked to lymph nodes via migration of antigen-presenting cells, and via drainage through lymphatic vessels in the meningeal sinuses (2). In the lymphatic tissues, antigen presentation and T-cell priming takes place. This interaction is tightly regulated by a multitude of co-inhibitory (CTLA4) and co-stimulatory (CD80, CD86, CD28) immune checkpoint molecules, and could be modulated by specific therapeutic antibodies, such as the CTLA4 inhibitor ipilimumab (3). Activated T cells reach the tumour via the blood stream and migration through the blood–brain or blood–tumour barrier (4). Tumour-associated immunosuppressive factors, including immune checkpoint molecules, inhibit tumour cell destruction by T cells. PDL1 is expressed on tumour cells and microglia and inhibits T cells via binding to PD1. PD1–PDL1 inhibitors (for example, nivolumab, pembrolizumab) block this immunosuppressive mechanism and thereby increase tumour cell lysis by lymphocytes (5). Abbreviations: CTLA4, cytotoxic T-lymphocyte-associated antigen 4; MHC, major histocompatibility complex; PDL1, programmed cell death 1 ligand 1; PD1, programmed cell death protein 1; TCR, T-cell receptor.