Abstract
Background
The rapid reduction in plasma osmolality during hemodialysis (HD) may induce temporary gradients that promote the movement of water from the extracellular to the intracellular compartment, predisposing to development of intra-dialytic hypotension (IDH).
Study Design
Observational cohort study.
Setting & Participants
The cohort comprises 3,142 prevalent patients receiving thrice-weekly HD from a single dialysis provider organization.
Predictor
Pre-dialysis calculated plasma osmolarity (calculated after the two-day interval as 2*serum sodium + serum urea nitrogen/2.8 + serum glucose/18).
Outcome
Magnitude of systolic blood pressure (SBP) decline (pre-dialysis SBP - nadir intra-dialytic SBP) and risk of IDH (SBP decline >35 or nadir SBP <90 mmHg).
Measurements
Unadjusted and multivariable adjusted generalized linear models were fit to estimate the association of calculated osmolarity with intra-dialytic SBP decline and the odds of developing IDH.
Results
The mean age of participants was 62.6 ± 15.2 (SD) years; 57.1% were male; 61.0% were diabetic. The mean pre-dialysis calculated osmolarity was 306.4 ± 9.5 mOsm/kg. After case-mix adjustment, each 10-mOsm/L increase in predialysis calculated osmolarity was associated with 1.48 (95% CI, 0.86–2.09) mm Hg (p<0.001) greater decline in intradialytic SBP and 10% greater odds of IDH (OR, 1.10; 95% CI, 1.05–1.15). In adjusted models, lower predialysis sodium, higher SUN and higher serum glucose were associated with greater decline in intra-dialytic SBP.
Limitations
Measured serum osmolality, timing of changes in intra-dialytic osmolality, dialysate osmolality and dialysate temperature were not available.
Conclusions
Higher pre-dialysis calculated osmolarity is associated with greater decline in intra-dialytic SBP and greater risk of IDH in long-term HD patients. Strategies to minimize rapid shifts in osmolality should be tested prospectively to minimize excess SBP decline in susceptible patients.
Keywords: intra-dialytic hypotension, osmolality, hemodialysis
Intra-dialytic hypotension (IDH)—defined as significant and abrupt decline in systolic blood pressure (SBP) during the hemodialysis (HD) procedure that causes symptoms and/or requires an intervention—is estimated to affect up to one third of outpatient HD sessions.1–3 IDH events are associated with a greater incidence of myocardial stunning,4 cerebral atrophy5 and greater all-cause mortality.6 The underlying pathogenesis of intra-dialytic SBP decline is likely to be multi-factorial, including the presence of autonomic neuropathy,7 higher rates of ultrafiltration8 and temporary changes in osmolality induced by the HD procedure itself.9
The mean pre-dialysis serum osmolality in HD patients is reported to range from 291–339 mOsm/kg, and may decline by up to 33 mOsm/kg during dialysis,10–13 primarily due to changes in sodium (SNa), glucose, and urea (the three major constituents of serum osmolality). We previously reported that greater rates of decline in serum urea nitrogen (SUN) during HD are associated with a greater risk of developing IDH.9 Previous studies have reported that the use of higher dialysate sodium (DNa)14 and other hyperosmolar substances15–16 may limit the development of transient osmotic gradients and thereby promote intra-dialytic hemodynamic stability.
We wished to determine the association of pre-dialysis calculated osmolarity with the magnitude of decline in intra-dialytic SBP. We hypothesized that higher pre-dialysis calculated osmolarity would be associated with greater intra-dialytic SBP decline.
Methods
Study Design and Population
The study protocol was deemed exempt by the Partners Healthcare Institutional Review Board. We performed a non-concurrent cohort study of prevalent patients receiving HD in Satellite Healthcare facilities in 2012. Patients became eligible for participation on the earliest date within this time period on which they were at least 18 years of age and had been receiving HD for greater than 180 days (n=3722). Those not on thrice weekly HD (n=291), those with session length >5 hours (n=23) and those without available variables to calculate the pre-dialysis calculated osmolarity (n=231) or intra-dialytic SBP decline (SBP readings <40 or >240 mmHg or missing; n=18) were excluded. As the majority of laboratory values (97%) were measured on the first dialysis day of the week (i.e. Monday or Tuesday), we excluded patients with blood drawn on other days (n=17). The final cohort consisted of 3,142 individuals and 21,646 HD sessions.
Exposures and Outcomes
The primary exposure of interest was the calculated osmolarity, equal to (2*serum sodium) + (SUN/2.8) + (serum glucose/18). Secondary exposures of interest included pre-dialysis serum sodium, glucose and SUN (recorded from routine monthly blood draws). The primary outcome was the magnitude of intra-dialytic SBP decline, defined as the pre-dialysis SBP less the nadir intra-dialytic SBP. Routine blood pressures were measured every 30 minutes during dialysis; the median number of measurements per session was eight. The secondary outcome of interest was the development of intra-dialytic hypotension, defined as a decline in SBP >35 mmHg, or any intra-dialytic SBP <90 mmHg.
Study Data
Demographic data including sex, race, co-morbid conditions and age were recorded at baseline. Dialysis treatment and hemodynamic parameters were recorded at each individual HD session; only sessions with corresponding laboratory data were included in the analyses. Laboratory measurements were obtained prior to the first HD session of the week (after the two-day inter-dialytic interval) on a monthly basis and processed in a central laboratory. All patients were dialyzed against a dialysate glucose concentration of 100 mg/dL.17 The DNa concentrations used in these treatments ranged from 126 to 148 mmol/L, including 16.1% that utilized sodium modeling algorithms.
Statistical Analysis
Continuous variables were examined graphically and recorded as means ± standard deviations for normally distributed data, or medians with inter-quartile ranges for non-normally distributed data. Comparisons were made using analysis of variance or Kruskal-Wallis tests as appropriate. Categorical variables were examined by frequency distribution, recorded as proportions and comparisons made using the χ2 test.
Initially, unadjusted generalized linear regression models (using a normal distribution and identity link function) were fit to assess the association of calculated osmolarity with intra-dialytic SBP decline. Subsequently, in Model 1, adjustment was made for age, sex (male vs. female), race (black vs. non-black), diabetes, ischemic heart disease, congestive heart failure, access type (fistula, graft, catheter), pre-dialysis SBP and ultrafiltration rate (UFR in mL/kg/h). Model 2 was adjusted for the same variables as Model 1, in addition to pre-dialysis serum calcium, albumin, and bicarbonate. Subsequently, further models were fit, using a binomial distribution and logit link function, to determine the association of calculated osmolarity with the odds of developing intra-dialytic hypotension. As extreme hyperglycemia may influence the calculated osmolarity, sensitivity analyses were performed when excluding those with pre-dialysis glucose concentrations >132 mg/dL. In order to evaluate the individual components that contribute to the calculated osmolarity, secondary analyses were performed in the manner already described to determine the association of pre-dialysis SNa, glucose and SUN with the magnitude of intra-dialytic SBP decline and odds of developing IDH. Finally, via the inclusion of cross-product terms, exploratory analyses were performed to assess for effect modification of the association of calculated osmolarity with SBP decline according to higher (>140 mmol/L or modeling) versus lower (≤140 mmol/L) DNa use and UFR, with sub-group analyses presented for lower and higher DNa use and tertiles of UFR.
Covariates for all models were selected on the basis of clinical and biological plausibility, without use of probabilistic selection criteria. Nominal two-sided p-values of <0.05 were considered statistically significant. Analyses were performed using Stata MP 13.1 (College Station, TX).
Results
Study Participants
The mean age of the patients was 62.6 ± 15.2 (standard deviation) years; 12.9% were black and 61.0% were diabetic. Individuals in the highest tertile of baseline calculated osmolarity tended to be younger and male and have higher pre-dialysis SNa, SUN, serum glucose and albumin, but lower pre-dialysis serum bicarbonate concentrations (Table 1). Those in the highest tertile of calculated osmolarity also tended to have higher pre-dialysis SBP, greater ultrafiltration volume and UFR, greater intra-dialytic SBP decline and greater frequency of IDH (Table 2). During a median follow up time of 5.3 months, the mean pre-dialysis calculated osmolarity was 306.4 ± 9.5 mOsm/kg.
Table 1.
Baseline characteristics of the cohort according to tertiles of pre-dialysis calculated osmolarity
| Total (N=3,142) | Calculated Osmolarity Tertile | Pa | |||
|---|---|---|---|---|---|
| 1 (n=1,048) | 2 (n=1,049) | 3 (n=1,045) | |||
| Age (y) | 62.6 ± 15.2 | 64.0 ± 14.9 | 62.5 ± 14.9 | 61.4 ± 15.5 | <0.001 |
| Male sex | 1795, 57.1 | 555, 53.0 | 596, 56.8 | 644, 61.6 | <0.001 |
| Black race | 406, 12.9 | 145, 13.8 | 141, 13.4 | 120, 11.5 | 0.2 |
| Diabetes | 1918, 61.0 | 624, 59.5 | 643, 61.3 | 651, 62.3 | 0.4 |
| IHD | 529, 16.8 | 181, 17.3 | 174, 16.6 | 174, 16.6 | 0.9 |
| CHF | 772, 24.6 | 252, 24.0 | 274, 26.1 | 246, 23.5 | 0.4 |
| Access type | 0.2 | ||||
| AVF | 1965, 62.6 | 639, 61.0 | 659, 62.8 | 667, 63.8 | |
| AVG | 570, 18.1 | 200, 19.1 | 202, 19.3 | 168, 16.1 | |
| Catheter | 607, 19.3 | 209, 19.9 | 188, 17.9 | 210, 20.1 | |
| Session Length | 0.4 | ||||
| ≤180 min | 1818, 57.9 | 585, 55.8 | 611, 58.3 | 622, 59.5 | |
| 181–209 min | 781, 24.9 | 270, 25.8 | 252, 24.0 | 259, 24.8 | |
| >209 min | 543, 17.2 | 193, 18.4 | 186, 17.7 | 164, 15.7 | |
| Postdialysis weight (kg) | 76.2 ± 20.9 | 76.4 ± 21.4 | 76.3 ± 20.3 | 76.0 ± 20.9 | 0.9 |
| Serum Sodium (mmol/L) | 136.8 ± 3.2 | 134.9 ± 3.1 | 137.1 ± 2.6 | 138.4 ± 2.9 | <0.001 |
| Serum Albumin (g/dL) | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.4 | <0.001 |
| SUN (mg/dL) | 65.9 ± 19.8 | 51.6 ± 14.6 | 64.1 ± 14.0 | 82.0 ± 17.4 | <0.001 |
| Serum Bicarbonate (mmol/L) | 24.0 ± 3.3 | 24.3 ± 3.4 | 23.9 ± 3.3 | 23.7 ± 3.4 | <0.001 |
| Serum Glucose (mg/dL) | 142.3 ± 70.2 | 128.4 ± 57.3 | 145.3 ± 71.8 | 153.3 ± 77.7 | <0.001 |
| Calculated osmolarity (mOsm/L) | 305.1 ± 9.5 | 295.0 ± 5.7 | 305.2 ± 2.2 | 315.2 ± 5.3 | <0.001 |
Note: Values for categorical variables are given as number (percentages); values for continuous variables, as mean ± standard deviation. Conversion factors for units: glucose in mg/dL to mmol/L, ×0.05551; SUN in mg/dL to mmol/L, ×0.357.
IHD, ischemic heart disease; CHF, congestive heart failure; AVF, arteriovenous fistula; AVG, arteriovenous graft; SUN, serum urea nitrogen.
P values refer to testing the null of no difference between groups, using analysis of variance for continuous variables and χ2 tests for categorical variables.
Table 2.
Baseline hemodialysis treatment blood pressure characteristics according to tertiles of pre-dialysis calculated osmolarity
| Total (N=3,142) | Calculated Osmolarity Tertile | Pa | |||
|---|---|---|---|---|---|
| 1 (n=1,048) | 2 (n=1,049) | 3 (n=1,045) | |||
| Pre-dialysis SBP (mmHg) | 150.4 ± 26.1 | 148.0 ± 26.9 | 150.7 ± 25.7 | 152.4 ± 25.6 | <0.001 |
| Minimum Intradialytic SBP (mmHg) | 110.0 ± 26.9 | 110.7 ± 27.6 | 109.2 ± 26.3 | 110.2 ± 26.8 | 0.4 |
| Postdialysis SBP (mmHg) | 135.5 ± 24.3 | 136.4 ± 24.8 | 135.0 ± 24.3 | 135.1 ± 24.0 | 0.3 |
| SBP decline (mmHg) | 40.5 ± 28.5 | 37.5 ± 29.2 | 41.7 ± 27.6 | 42.4 ± 28.6 | <0.001 |
| SBP variabilityb (mmHg) | 15.8 ± 8.4 | 15.5 ± 8.2 | 16.0 ± 8.4 | 15.9 ± 8.5 | 0.4 |
| UF volume (L) | 3.0 ± 1.2 | 3.0 ± 1.3 | 3.0 ± 1.2 | 3.1 ± 1.1 | 0.001 |
| UFR (ml/kg/h) | 12.8 ± 4.9 | 12.2 ± 5.2 | 12.8 ± 4.7 | 13.4 ± 4.8 | <0.001 |
| IDH | 1,733 (55.2) | 542 (51.7) | 598 (57.0) | 593 (56.8) | 0.02 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation.
Hemodialysis-associated blood pressure variables (± standard deviation) according to baseline dialysate sodium use (≤140 mmol/L vs. >140 mmol/L or modeling).
Abbreviations and definitions: SBP, systolic blood pressure; UF, ultrafiltration; UFR, ultrafiltration rate; IDH, intradialytic hypotension (defined as SBP decline >35 mmHg or nadir intra-dialytic SBP <90 mmHg).
P values refer to testing the null of no difference between groups, using analysis of variance for continuous variables and χ2 tests for categorical variables.
SBP variability was calculated as the standard deviation of all intra-dialytic SBP measurements.
Predialysis Calculated Osmolarity and Intradialytic SBP Decline
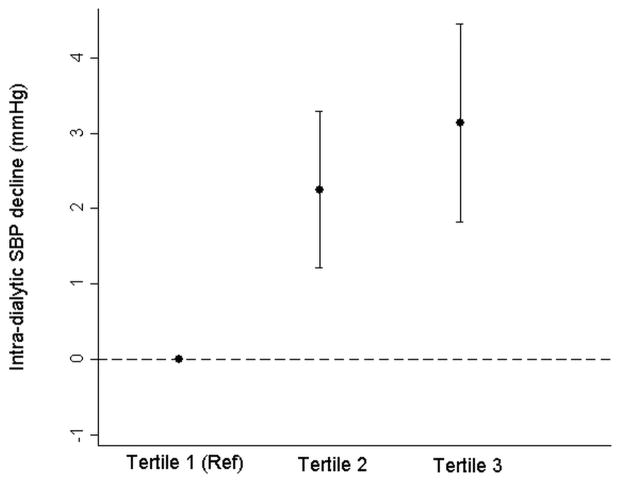
In unadjusted models, each 10-mOsm/L increment in the pre-dialysis calculated osmolarity was associated with a 2.22 (95% confidence interval [CI], 1.51–2.93) mm Hg greater decline in intra-dialytic SBP. In the fully adjusted model (Model 2), the association was attenuated, but remained statistically significant (1.48 [95% CI, 0.86–2.09] mm Hg), even after exclusion of patients with pre-dialysis serum glucose > 132 mg/dL (0.98 [95% CI, 0.23–1.70] mmHg [Table 3]). Effect estimates were qualitatively and quantitatively similar after individual additional adjustment for categories of blood flow, dialysate bicarbonate and dialysate calcium (data not shown). When analyzed in tertiles of calculated osmolarity, a monotonic association was apparent (Figure 1). Similar patterns of association were evident when IDH was considered as the outcome of interest (Table 3).
Table 3.
Association of predialysis calculated osmolarity with intra-dialytic SBP decline and hypotension
| Intradialytic SBP decline per 10-mOsm/L greater predialysis calculated osmolarity | Odds of hypotension per 10-mOsm/L greater predialysis calculated osmolarity | |
|---|---|---|
| Unadjusted model | 2.22 (1.51 to 2.93) mm Hg; P<0.001 | 1.10 (1.05 to 1.15); P<0.001 |
| Model 1 | 1.28 (0.68 to 1.88) mm Hg; P<0.001 | 1.08 (1.03 to 1.13); P=0.001 |
| Model 2 | 1.48 (0.86 to 2.09) mm Hg; P<0.001 | 1.10 (1.05 to 1.15); P<0.001 |
| Model 2A | 0.98 (0.23 to 1.70) mm Hg; P=0.01 | 1.06 (1.00 to 1.12); P=0.06 |
| Model 3 | 1.48 (0.87 to 2.10) mm Hg; P<0.001 | 1.10 (1.05 to 1.15); P<0.001 |
| Lower dialysate sodium | 1.71 (1.02 to 2.39) mm Hg; P<0.001 | 1.11 (1.06 to 1.18); P<0.001 |
| Higher dialysate sodium | 0.70 (−0.59 to 2.00) mm Hg; P=0.3 | 1.02 (0.93 to 1.13); P=0.6 |
Note: Values in parentheses are 95% confidence interval. Generalized linear models were fit to estimate the association of pre-dialysis calculated osmolality with intra-dialytic SBP decline or odds of intra-dialytic hypotension (decline in SBP >35 mmHg, or any intra-dialytic SBP <90 mmHg). Model 1 adjusted for age, sex, race (black versus non-black), diabetes, ischemic heart disease, congestive heart failure, access type (fistula, graft, catheter), pre-dialysis SBP and ultrafiltration rate. Model 2 adjusted for the same variables as Model 1 in addition to serum calcium, albumin, and bicarbonate. Model 2A excluded those with pre-dialysis serum glucose >132 mg/dL. Model 3 adjusted for the same variables as Model 2, in addition to dialysate sodium use (≤140 mmol/L vs. >140 mmol/L or modeling).
SBP, systolic blood pressure;
Figure 1.
Generalized linear models were fit to estimate the association of tertiles of pre-dialysis calculated osmolality with the magnitude of intra-dialytic SBP decline (Tertile 1 was taken as the reference), after adjusting for age, gender (male versus female), race (black versus non-black), diabetes, ischemic heart disease, congestive heart failure, access type (fistula, graft, catheter), pre-dialysis SBP, UFR, serum calcium, albumin, and bicarbonate.
Predialysis Serum Sodium, SUN, Glucose and Intradialytic SBP Decline
In secondary analyses, the associations of SNa, SUN and serum glucose with intra-dialytic SBP decline were examined. In the fully adjusted model (Model 2), lower SNa, higher SUN and higher glucose were independently associated with greater SBP decline (Table 4). After the exclusion of individuals with pre-dialysis glucose >132 mg/dL, the association of glucose with SBP decline became non-significant. Effect estimates were qualitatively and quantitatively similar after individual additional adjustment for categories of blood flow, dialysate bicarbonate and dialysate calcium (data not shown). Similar patterns of association were evident when IDH was considered as the outcome of interest (Table 5).
Table 4.
Association of pre-dialysis serum sodium, SUN and serum glucose and with intra-dialytic SBP decline
| Intradialytic SBP decline per 1-mmol/L greater predialysis serum sodium | Intradialytic SBP decline per 2.8-mg/dL greater predialysis SUN | Intradialytic SBP decline per 18-mg/dL greater predialysis serum glucose | |
|---|---|---|---|
| Unadjusted model | 0.00 (−0.20 to 0.20); P=0.9 | 0.33 (0.24 to 0.42); P<0.001 | 0.50 (0.33 to 0.67); P<0.001 |
| Model 1 | −0.25 (−0.43 to −0.08); P=0.01 | 0.31 (0.23 to 0.39); P<0.001 | 0.18 (0.03 to 0.33); P=0.02 |
| Model 2 | −0.22 (−0.40 to −0.04); P=0.02 | 0.33 (0.25 to 0.42); P<0.001 | 0.19 (0.04 to 0.34); P=0.01 |
| Model 2A | −0.26 (−0.48 to −0.05); P=0.02 | 0.29 (0.19 to 0.39); P<0.001 | −0.09 (−0.62 to 0.44); P=0.74 |
| Model 3 | −0.22 (−0.40 to −0.04); P=0.02 | 0.33 (0.25 to 0.42); P<0.001 | 0.20 (0.05 to 0.35); P=0.01 |
| Lower dialysate sodium | −0.14 (−0.35 to 0.06); P=0.2 | 0.34 (0.24 to 0.43); P<0.001 | 0.26 (0.10 to 0.42); P=0.002 |
| Higher dialysate sodium | −0.46 (−0.85 to −0.09); P=0.02 | 0.35 (0.15 to 0.54); P<0.001 | −0.07 (−0.42 to 0.27); P=0.7 |
Note: Values are provided in mm Hg; values in parentheses are 95% confidence intervals. Generalized linear models were fit to estimate the association of pre-dialysis sodium, SUN and glucose with intra-dialytic SBP decline. Model 1 additionally adjusted for age, sex, race (black versus non-black), diabetes, ischemic heart disease, congestive heart failure, access type (fistula, graft, catheter), pre-dialysis SBP and ultrafiltration rate. Model 2 adjusted for the same variables as Model 1 in addition to serum calcium, albumin, and bicarbonate. Model 2A excluded those with pre-dialysis serum glucose >132 mg/dL. Model 3 adjusted for the same variables as Model 2, in addition to dialysate sodium use (≤140 mmol/L vs. >140 mmol/L or modeling).
SBP, systolic blood pressure; SUN, serum urea nitrogen
Table 5.
Association of pre-dialysis serum sodium, SUN, and serum glucose with intra-dialytic hypotension
| Odds of intradialytic SBP per 1-mmol/L greater predialysis serum sodium | Odds of intradialytic SBP per 2.8-mg/dL greater predialysis SUN | Odds of intradialytic SBP per 18-mg/dL greater predialysis serum glucose | |
|---|---|---|---|
| Unadjusted | 0.99 (0.98 to 1.00); P=0.2 | 1.02 (1.01 to 1.02); P<0.001 | 1.03 (1.02 to 1.04); P<0.001 |
| Model 1 | 0.98 (0.96 to 0.99); P=0.001 | 1.02 (1.01 to 1.03); P<0.001 | 1.02 (1.00 to 1.03); P=0.01 |
| Model 2 | 0.98 (0.97 to 0.99); P=0.01 | 1.02 (1.02 to 1.03); P<0.001 | 1.02 (1.00 to 1.03); P=0.01 |
| Model 2A | 0.98 (0.96 to 0.99); P=0.01 | 1.02 (1.01 to 1.03); P<0.001 | 1.00 (0.96 to 1.05); P=0.9 |
| Model 3 | 0.98 (0.97 to 1.00); P=0.01 | 1.02 (1.02 to 1.03); P<0.001 | 1.02 (1.01 to 1.03); P<0.001 |
| Lower dialysate sodium | 0.99 (0.97 to 1.00); P=0.06 | 1.02 (1.02 to 1.03); P<0.001 | 1.02 (1.00 to 1.03); P=0.01 |
| Higher dialysate sodium | 0.97 (0.94 to 0.99); P=0.02 | 1.02 (1.00 to 1.03); P=0.02 | 1.02 (0.99 to 1.05); P=0.3 |
Note: Values in parentheses are 95% confidence intervals. Generalized linear models were fit to estimate the association of pre-dialysis sodium, BUN and glucose with intra-dialytic hypotension (decline in SBP >35 mmHg, or any intra-dialytic SBP <90 mmHg). Model 1 additionally adjusted for age, sex, race (black versus non-black), diabetes, ischemic heart disease, congestive heart failure, access type (fistula, graft, catheter), pre-dialysis SBP and ultrafiltration rate. Model 2 adjusted for the same variables as Model 1 in addition to serum calcium, albumin, and bicarbonate. Model 2A excluded those with pre-dialysis serum glucose >132 mg/dL. Model 3 adjusted for the same variables as Model 2, in addition to dialysate sodium use (≤140 mmol/L vs. >140 mmol/L or modeling).
SBP, systolic blood pressure; SUN, serum urea nitrogen
Exploratory Analyses
Addition of higher vs. lower DNa as a covariate to the fully adjusted model (Model 3) revealed no evidence of significant change to the point estimate of the association of calculated osmolarity with intra-dialytic SBP decline (1.48 [95% CI, 0.87–2.10] mm Hg). However, in sub-group analyses according to higher or lower DNa use, calculated osmolarity remained associated with hypotension in the lower DNa sub-group only (Table 3). In the analyses of SNa, SUN and serum glucose, only higher SUN remained consistently associated with SBP decline across DNa sub-groups (Tables 4 & 5).
In other exploratory analyses we found evidence for effect modification of the association of calculated osmolarity with SBP decline by UFR (p for interaction<0.001). In these analyses, greater calculated osmolarity only remained associated with greater SBP decline in the lower two tertiles of UFR, although the mean calculated osmolarity was lower in tertile 3 than in tertile 2 (Table 6).
Table 6.
Association of pre-dialysis calculated osmolarity with intra-dialytic SBP decline, according to tertiles of ultrafiltration rate
| UFR Tertile 1 | UFR Tertile 2 | UFR Tertile 3 | |
|---|---|---|---|
| Intradialytic SBP decline per 10-mOsm/L greater calculated Osmolarity (mm Hg) | 2.4 (1.5 to 3.3) | 1.7 (0.8 to 2.6) | 0.3 (−0.6 to 1.2) |
| P | <0.001 | <0.001 | 0.5 |
| Mean calculated osmolarity (mOsm/L) | 305.6±9.6 | 307.0±9.3 | 306.5±9.6 |
Note: Values in parentheses are 95% confidence intervals. Generalized linear models were fit to estimate the association of pre-dialysis calculated osmolarity with intra-dialytic SBP decline. Model 2 adjusted for age, sex, race (black versus non-black), diabetes, ischemic heart disease, congestive heart failure, access type (fistula, graft, catheter), pre-dialysis SBP, UFR, serum calcium, albumin, and bicarbonate.
UFR, ultrafiltration rate
Discussion
In this study of dialysis-associated SBP changes, we report that higher pre-dialysis calculated osmolarity, measured after the two-day inter-dialytic interval, is independently associated with greater decline in intra-dialytic SBP and greater odds of having IDH.
In normal physiological states, the calculated osmolarity, equal to (2*serum sodium) + (SUN/2.8) + (serum glucose/18), approximates the measured osmolality.. As sodium is the major contributor to the serum osmolality, one might expect higher pre-dialysis SNa to be associated with greater SBP decline. We found the opposite to be the case, but noted a significant negative correlation between glucose and SNa in our study (r=−0.32; P<0.001), suggesting that severe hyperglycemia may partially obscure the association of SNa with outcomes of interest. However, even after exclusion of those with higher glucose levels, the direction of association of SNa with SBP decline did not change. A more likely explanation, consistent with our current findings (correlation between SNa and UF volume: r= −0.18; P<0.001), may be that lower SNa associates with greater inter-dialytic weight gain18 (and perhaps lower solute intake), identifying those who are more likely to have intra-dialytic hypotension as a result of higher UFRs.19 Interestingly, we found that higher calculated osmolarity was not associated with greater SBP decline in the highest tertile of UFR, suggesting that in this particular sub-group, the risk of hypotension from a higher rate of fluid removal may outweigh any contribution from changes in osmolality. However, an alternative explanation may again be that greater interdialytic weight gain results in a lower pre-dialysis osmolality, obscuring any association with greater SBP decline. In support of this assertion, we reported that the pre-dialysis calculated osmolarity for the highest tertile of UFR was indeed lower than that of the middle tertile.
The relatively high calculated osmolarity in HD patients, compared with patients who have normal kidney function, largely reflects the contribution of higher SUN concentrations. In states of rapid flux (such as HD), it is possible that rapid urea clearance from the extracellular compartment may predispose to the generation of temporary osmotic gradients,20–23 promoting transcellular movement of water and resultant hypotension. Of note, higher pre-dialysis SUN may partially reflect better nutritional status in some hemodialysis patients24—therefore, it is important to point out that protein restriction is not to be advocated as a means to reduce SUN for the prevention of IDH. Knowledge related to the mechanisms of water and urea movement across body compartments continues to evolve25–26 and future experimental work will shed further light on this important area.
Higher blood glucose concentrations may also contribute to higher pre-dialysis osmolality measurements in long-term HD patients. The optimal dialysate glucose prescription is one that minimizes the risk of hypoglycemia, while also limiting exposure to excessively high glucose concentrations.27 However, in individuals with hyperglycemia, fixed lower dialysate glucose may actually lead to larger blood–dialysate gradients and a more rapid lowering of blood glucose during the HD procedure. Prior reports have demonstrated reduced frequency of IDH in diabetic patients when dialyzed against 200 mg/dL versus 100 mg/dL dialysate glucose.28 Our findings support the theory that larger blood–dialysate glucose concentration gradients may also predispose to greater intra-dialytic blood pressure decline.
Excess SBP decline during HD is a common occurrence and associated with worse all-cause and cardiovascular mortality, even in the absence of symptoms.29–30 Whether interventions to minimize rapid changes in osmolality that result in a 2– to 4–mm Hg lesser decline in SBP would lead to improved outcomes is not known, but these margins may be all the more important for end-organ perfusion in HD patients with stiff vessels that are dependent on maintenance of adequate perfusion pressure.31 It is therefore imperative to identify modifiable risk factors that may limit excessive drops in blood pressure during HD. One such example relates to the pressor effects of arginine vasopressin (AVP). Pre-dialysis AVP levels in HD patients are higher than those of the individuals without kidney disease (presumably reflecting the higher baseline osmolality). However, temporary lowering of plasma osmolality during HD may result in a blunted response of AVP to hypotension.32 On the other hand, minimizing the decline in serum osmolality during HD by the administration of hypertonic fluids has been shown to augment AVP release and promote blood pressure stability.10, 33–34 We previously reported that the rapidity of decline in SUN associated with greater odds of intra-dialytic hypotension in a post-hoc analysis of the HEMO (Hemodialysis) Study.9 This observation may also partly explain why isolated ultrafiltration (with minimal changes in plasma osmolality) tends to have less associated hypotension.35 Our current finding, that higher predialysis calculated osmolarity is associated with greater magnitude of SBP decline, is consistent with these observations.
There are numerous reports detailing interventions aimed at minimizing the rate of osmolality decline that may be useful in the treatment of patients prone to hypotensive events. Dialysis physicians initially increased the DNa concentration, finding that this reduced the frequency of dialysis-related symptoms16 and improved hemodynamic stability.36–37 Prior reports of increased thirst and inter-dialytic weight gain tempered the initial enthusiasm of these findings, 8, 37 but more recently the use of biofeedback-controlled sodium profiling appears to have less associated interdialytic weight gain. 38 Whether treatment, or aggressive focus on prevention, of pre-dialysis hyperglycemia (taking care to avoid hypoglycemia) would attenuate the risk of IDH is currently unknown. Other strategies include the administration of hypertonic mannitol and other hypertonic solutions. 10, 15, 34, 39
In our analyses, we did not find any evidence for effect modification or confounding of our model estimates by the DNa concentration. In this regard, it must be remembered that higher DNa is generally prescribed for patients who are already known to suffer from IDH – this confounding by indication has been reported previously and may actually be expected to lead to an association of higher DNa use with greater SBP decline.40 Furthermore, as higher DNa may not be expected to associate with pre-dialysis calculated osmolarity, but rather to associate with less rapid decline in intra-dialytic osmolality, it is not surprising that it does not behave as a confounding variable in our analyses. However, it is interesting to note that greater calculated osmolarity only remained significantly associated with hypotension in the lower DNa subgroup. Although underpowered, and potentially subject to significant confounding as explained above, this observation suggests that the association of osmolality with intra-dialytic SBP decline may be altered by the use of differing DNa concentrations. Unfortunately we did not have actual pre- and post-HD samples to determine the measured osmolality changes in individual subjects.
We and others have reported a potential survival benefit for certain individuals dialyzing against higher DNa,41–43 although several large dialysis organizations have put forward proposals to lower the DNa concentration, based largely on observational evidence.44 This stance has already met with some opposition.45 It therefore seems imperative to perform interventional trials to identify best practices for the safe and effective delivery of HD.
The strengths of this study include the availability of a relatively large number of patients with detailed intra-dialytic hemodynamic data from individual HD sessions. There are, however, several limitations that warrant discussion. As this is an observational study, there remains the possibility of residual confounding from variables not considered, or insufficient adjustment from those that were. For example, data limitations prevented us from taking into account dialysate temperature, measured osmolality and medication use in our analyses. The demographic make-up of our sample differs from that of the general US population (lower proportion of African-Americans), thus limiting the broader generalizability of our findings. It is notable that this study analyzed laboratory data from the first HD day of the week (i.e. Monday or Tuesday) and therefore followed the longer two-day inter-dialytic interval, when pre-dialysis osmolality may be expected to be highest. Within the current confines of thrice-weekly HD for the vast majority of patients in the United States, it is possible that alternative dialysis prescriptions could be required for different days of the week.
In conclusion, higher pre-dialysis calculated osmolarity, due primarily to elevations in urea and glucose, is associated with greater magnitude of decline in intra-dialytic SBP and greater odds of having a hypotensive event. Strategies to limit pre-dialysis elevations in osmolality (such as treatment or avoidance of hyperglycemia before dialysis) and strategies to minimize the rapidity of osmotic shifts (such as isolated ultrafiltration, longer dialysis session length or greater HD frequency) should be tested to minimize the frequency and magnitude of SBP decline and its associated morbidity and mortality.
Acknowledgments
The authors thank Satellite Healthcare Inc for access to the data used in this study. Satellite Healthcare Inc had no role in the study design; collection, analysis, and interpretation of data; writing the report or the decision to submit the report for publication. This manuscript does not necessarily reflect the views or opinions of Satellite Healthcare Inc.
Support: Dr Mc Causland is supported by the American Heart Association National Fellow-to-Faculty Award (2014–2019). Dr Waikar is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants DK093574, DK075941, and U01DK085660.
Footnotes
Financial Disclosure: Dr Waikar served as a consultant to AbbVie, CVS Caremark, Harvard Clinical Research Institute, and Takeda; provided expert testimony or consultation for litigation related to nephrogenic systemic fibrosis (GE Healthcare) and mercury exposure; and has received research grants from the NIDDK, Genzyme, Merck, Otsuka, Pfizer, and Satellite Healthcare. Dr Mc Causland declares that he has no other relevant financial interests.
Contributions: Research idea and study design: FRMC; data acquisition: FRMC; data analysis/interpretation: FRMC, SSW; statistical analysis: FRMC, SSW; supervision or mentorship: SSW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. FRMC takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bos WJ, Bruin S, van Olden RW, et al. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis. 2000;35:819–826. doi: 10.1016/s0272-6386(00)70250-2. [DOI] [PubMed] [Google Scholar]
- 2.Boon D, van Montfrans GA, Koopman MG, Krediet RT, Bos WJ. Blood pressure response to uncomplicated hemodialysis: the importance of changes in stroke volume. Nephron Clin Pract. 2004;96:c82–87. doi: 10.1159/000076745. [DOI] [PubMed] [Google Scholar]
- 3.Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol. 2008;19:8–11. doi: 10.1681/ASN.2007091006. [DOI] [PubMed] [Google Scholar]
- 4.Owen PJ, Priestman WS, Sigrist MK, et al. Myocardial contractile function and intradialytic hypotension. Hemodial Int. 2009;13:293–300. doi: 10.1111/j.1542-4758.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 5.Mizumasa T, Hirakata H, Yoshimitsu T, et al. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract. 2004;97:c23–30. doi: 10.1159/000077592. [DOI] [PubMed] [Google Scholar]
- 6.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 7.Kersh ES, Kronfield SJ, Unger A, Popper RW, Cantor S, Cohn K. Autonomic insufficiency in uremia as a cause of hemodialysis-induced hypotension. N Engl J Med. 1974;290:650–653. doi: 10.1056/NEJM197403212901203. [DOI] [PubMed] [Google Scholar]
- 8.Song JH, Park GH, Lee SY, Lee SW, Kim MJ. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol. 2005;16:237–246. doi: 10.1681/ASN.2004070581. [DOI] [PubMed] [Google Scholar]
- 9.Mc Causland FR, Brunelli SM, Waikar SS. Dialysis Dose and Intradialytic Hypotension: Results from the HEMO Study. Am J Nephrol. 2013;38:388–396. doi: 10.1159/000355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu K, Kurosawa T, Sanjo T. Effect of hyperosmolality on vasopressin secretion in intradialytic hypotension: a mechanistic study. Am J Kidney Dis. 2008;52:294–304. doi: 10.1053/j.ajkd.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Cernaro V, Lacquaniti A, Lorenzano G, et al. Apelin, plasmatic osmolality and hypotension in dialyzed patients. Blood Purif. 2012;33:317–323. doi: 10.1159/000337104. [DOI] [PubMed] [Google Scholar]
- 12.Stabellini G, Bosi GP, Valeno V, et al. Relation between the osmolality trend and ornithynedecarboxylase activity in red blood cells of uremic patients during hemodialytic treatment. Biomed Pharmacother. 1998;52:166–168. doi: 10.1016/s0753-3322(98)80206-0. [DOI] [PubMed] [Google Scholar]
- 13.Fasanella d’Amore T, Wauters JP, Waeber B, Nussberger J, Brunner HR. Response of plasma vasopressin to changes in extracellular volume and/or plasma osmolality in patients on maintenance hemodialysis. Clin Nephrol. 1985;23:299–302. [PubMed] [Google Scholar]
- 14.Dheenan S, Henrich WL. Preventing dialysis hypotension: a comparison of usual protective maneuvers. Kidney Int. 2001;59:1175–1181. doi: 10.1046/j.1523-1755.2001.0590031175.x. [DOI] [PubMed] [Google Scholar]
- 15.Canzanello VJ, Hylander-Rossner B, Sands RE, Morgan TM, Jordan J, Burkart JM. Comparison of 50% dextrose water, 25% mannitol, and 23.5% saline for the treatment of hemodialysis-associated muscle cramps. ASAIO Trans. 1991;37:649–652. [PubMed] [Google Scholar]
- 16.Port FK, Johnson WJ, Klass DW. Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney Int. 1973;3:327–333. doi: 10.1038/ki.1973.51. [DOI] [PubMed] [Google Scholar]
- 17.Munoz Mendoza J, Bayes LY, Sun S, Doss S, Schiller B. Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: a quality improvement study. Am J Kidney Dis. 2011;58:956–963. doi: 10.1053/j.ajkd.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecking M, Karaboyas A, Antlanger M, et al. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38:78–90. doi: 10.1159/000353104. [DOI] [PubMed] [Google Scholar]
- 19.Flythe JE, Kunaparaju S, Dinesh K, Cape K, Feldman HI, Brunelli SM. Factors associated with intradialytic systolic blood pressure variability. Am J Kidney Dis. 2012;59:409–418. doi: 10.1053/j.ajkd.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Arieff AI, Massry SG, Barrientos A, Kleeman CR. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int. 1973;4:177–187. doi: 10.1038/ki.1973.100. [DOI] [PubMed] [Google Scholar]
- 21.Pappius HM, Oh JH, Dossetor JB. The effects of rapid hemodialysis on brain tissues and cerebrospinal fluid of dogs. Can J Physiol Pharmacol. 1967;45:129–147. doi: 10.1139/y67-014. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy AC, Linton AL, Eaton JC. Urea levels in cerebrospinal fluid after haemodialysis. Lancet. 1962;1:410–411. doi: 10.1016/s0140-6736(62)91365-x. [DOI] [PubMed] [Google Scholar]
- 23.Silver SM, Sterns RH, Halperin ML. Brain swelling after dialysis: old urea or new osmoles? Am J Kidney Dis. 1996;28:1–13. doi: 10.1016/s0272-6386(96)90124-9. [DOI] [PubMed] [Google Scholar]
- 24.Bergstrom J. Nutrition and mortality in hemodialysis. J Am Soc Nephrol. 1995;6:1329–1341. doi: 10.1681/ASN.V651329. [DOI] [PubMed] [Google Scholar]
- 25.Bhave G, Neilson EG. Body Fluid Dynamics: Back to the Future. J Am Soc Nephrol. 2011;2011:27. doi: 10.1681/ASN.2011080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinh-Trang-Tan MM, Cartron JP, Bankir L. Molecular basis for the dialysis disequilibrium syndrome: altered aquaporin and urea transporter expression in the brain. Nephrol Dial Transplant. 2005;20:1984–1988. doi: 10.1093/ndt/gfh877. [DOI] [PubMed] [Google Scholar]
- 27.Burmeister JE, Scapini A, da Rosa Miltersteiner D, da Costa MG, Campos BM. Glucose-added dialysis fluid prevents asymptomatic hypoglycaemia in regular haemodialysis. Nephrol Dial Transplant. 2007;22:1184–1189. doi: 10.1093/ndt/gfl710. [DOI] [PubMed] [Google Scholar]
- 28.Simic-Ogrizovic S, Backus G, Mayer A, Vienken J, Djukanovic L, Kleophas W. The influence of different glucose concentrations in haemodialysis solutions on metabolism and blood pressure stability in diabetic patients. Int J Artif Organs. 2001;24:863–869. [PubMed] [Google Scholar]
- 29.Park J, Rhee CM, Sim JJ, et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013;84:795–802. doi: 10.1038/ki.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of Mortality Risk with Various Definitions of Intradialytic Hypotension. J Am Soc Nephrol. 2014;30:2014020222. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubin R, Owens C, Gasper W, Ganz P, Johansen K. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodial Int. 2011;15:350–358. doi: 10.1111/j.1542-4758.2011.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ettema EM, Zittema D, Kuipers J, et al. Dialysis hypotension: a role for inadequate increase in arginine vasopressin levels? A systematic literature review and meta-analysis. Am J Nephrol. 2014;39:100–109. doi: 10.1159/000358203. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu K, Kurosawa T, Ishikawa R, Sanjo T. Vasopressin secretion by hypertonic saline infusion during hemodialysis: effect of cardiopulmonary recirculation. Nephrol Dial Transplant. 2011;2011:7. doi: 10.1093/ndt/gfr272. [DOI] [PubMed] [Google Scholar]
- 34.Henrich WL, Woodard TD, Blachley JD, Gomez-Sanchez C, Pettinger W, Cronin RE. Role of osmolality in blood pressure stability after dialysis and ultrafiltration. Kidney Int. 1980;18:480–488. doi: 10.1038/ki.1980.161. [DOI] [PubMed] [Google Scholar]
- 35.Rouby JJ, Rottembourg J, Durande JP, Basset JY, Legrain M. Importance of the plasma refilling rate in the genesis of hypovolaemic hypotension during regular dialysis and controlled sequential ultrafiltration-haemodialysis. Proc Eur Dial Transplant Assoc. 1978;15:239–244. [PubMed] [Google Scholar]
- 36.Locatelli F, Covic A, Chazot C, Leunissen K, Luno J, Yaqoob M. Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant. 2004;19:785–796. doi: 10.1093/ndt/gfh102. [DOI] [PubMed] [Google Scholar]
- 37.Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. Am J Kidney Dis. 1997;29:669–677. doi: 10.1016/s0272-6386(97)90118-9. [DOI] [PubMed] [Google Scholar]
- 38.Locatelli F, Stefoni S, Petitclerc T, et al. Effect of a plasma sodium biofeedback system applied to HFR on the intradialytic cardiovascular stability. Results from a randomized controlled study. Nephrol Dial Transplant. 2012;2012:4. doi: 10.1093/ndt/gfs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mc Causland FR, Prior LM, Heher E, Waikar SS. Preservation of Blood Pressure Stability with Hypertonic Mannitol during Hemodialysis Initiation. Am J Nephrol. 2012;36:168–174. doi: 10.1159/000341273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecking M, Karaboyas A, Rayner H, et al. Dialysate sodium prescription and blood pressure in hemodialysis patients. Am J Hypertens. 2014;27:1160–1169. doi: 10.1093/ajh/hpu040. [DOI] [PubMed] [Google Scholar]
- 41.Mc Causland FR, Brunelli SM, Waikar SS. Dialysate sodium, serum sodium and mortality in maintenance hemodialysis. Nephrol Dial Transplant. 2011;27:1613–1618. doi: 10.1093/ndt/gfr497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecking M, Karaboyas A, Saran R, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2012;59:238–248. doi: 10.1053/j.ajkd.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Hecking M, Karaboyas A, Saran R, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner DE, Brunelli SM, Hunt A, et al. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis. 2014;64:685–695. doi: 10.1053/j.ajkd.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Port F, Hecking M, Karaboyas A, Pisoni R, Robinson B. Current evidence argues against lowering the dialysate sodium. Nephrol News Issues. 2013;27:18–21. [PubMed] [Google Scholar]