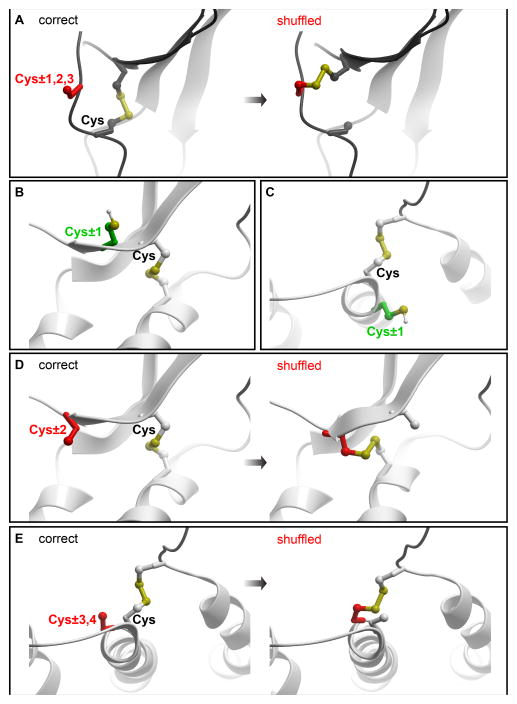
Figure 3.
Representative geometries of polypeptide chains where introduction of a cysteine poses high risk of intramolecular disulfide shuffling. (A) within 1–3 residues from a native cysteine in a loop without defined secondary structure. (B–C) within an α-helix and a β-strand, positions proximal to a native cysteine are generally safe. Instead, positions two residues up or down from a native cysteine within a β-strand (D) or 3–4 residues up or down from a native cysteine within an α-helix (E) are subject to disulfide shuffling liability.