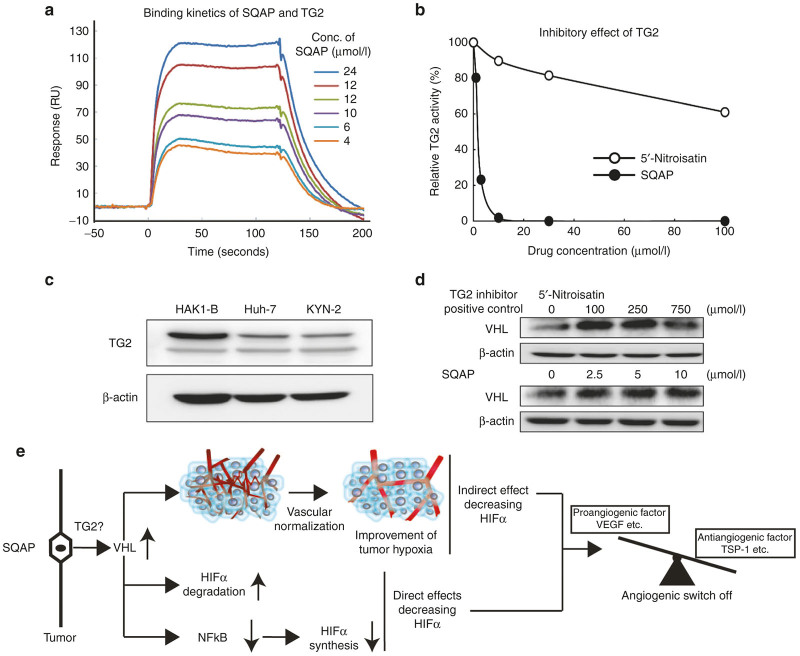
Figure 6.
Sulfoquinovosyl-acylpropanediol (SQAP) directly binds with and inhibits TG2 activity. (a) Binding analysis of TG2 with SQAP. An SPR analysis for the binding between SQAP and human TG2 was performed. SQAP was injected over flow cells on the immobilized TG2. The background resulting from the injection of running buffer was subtracted from the data before plotting. Resonance units (RU) were generated by subtraction of the background signal generated simultaneously on the control flow cell. (b) Inhibition of tissue transglutaminase activity. SQAP (filled circle) strongly inhibited the activity of TG2 compared to 5’-Nitroisatin (open circle). The horizontal axis indicates the concentration of drugs, and the vertical axis indicates the relative activity. (c) Western blots of TG2 in HAK1-B, Huh-7 and KYN-2. All HCC cell line we used expresses TG2. Cells were incubated under normoxia for 24 hours and then lysed with radioimmunoprecipitation buffer. (d) Western blots of pVHL in HAK1-B exposed different concentration of 5’-Nitroisatin and SQAP. 5’-Nitroisatin was used as a positive control for TG2 inhibitor. HAK1-B was incubated with different dose of 5’-Nitroisatin (100, 250, and 750 μmol/l) and SQAP (2.5, 5.0, and 10 μmol/l) under normoxia for 24 hours and then lysed with radioimmunoprecipitation buffer. (e) Summary of the multiple mechanisms decreasing HIFα of SQAP. SQAP upregulates tumor pVHL. Upregulated pVHL accelerates HIFα degradation. Moreover, upregulated pVHL decreases NFκB expression, resulting in decrease of HIFα synthesis. In addition to these direct effects decreasing HIFα, upregulated pVHL improves tumor hypoxia by induction of vascular normalization, which contributes to indirect decrease of HIFα proteins. All mechanisms contribute to induction of “angiogenic switch off” in tumor. SQAP directly binds and inhibits tumor TG2, implying that SQAP upregulates pVHL via inhibition of TG2.