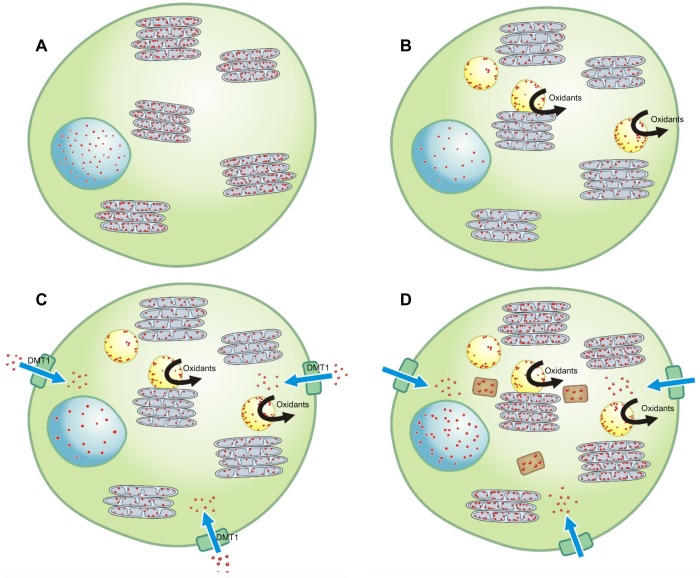
Figure 1.
Normally, a homeostasis of iron (designated by the small red dots) exists in a cell with the metal present at a concentration sufficient to meet structural and metabolic requirements; this includes the nucleus and mitochondria (designated by the blue circular and gray ovoid structures, respectively) (A). Introduction of an environmental chemical (designated by the yellow spherical structures) disrupts iron homeostasis as it, or a catabolic product, complexes the available iron, causing a functional deficiency of the metal in the cell (B). In response to a reduction in intracellular iron, the cell generates superoxide as a ferrireductant and upregulates importers (eg, DMT1) in an attempt to reacquire requisite metal (C). In addition, the complex of the environmental chemical with the iron may support electron transport, and oxidant generation may directly result from the reactions of this product with the available reductant and hydrogen peroxide (C). Oxidative stress activates cell signaling and transcription factors and will provoke a release of mediators initiating inflammation, fibrosis, and apoptosis (C). If the cell is effective in altering its iron homeostasis by increasing iron delivery, some portion of the metal will be stored in the protein ferritin (designated by the brown rectangular structures) (D). The result is an adequate level of metal available to the cell, including the environmental chemical, for continued survival and function.