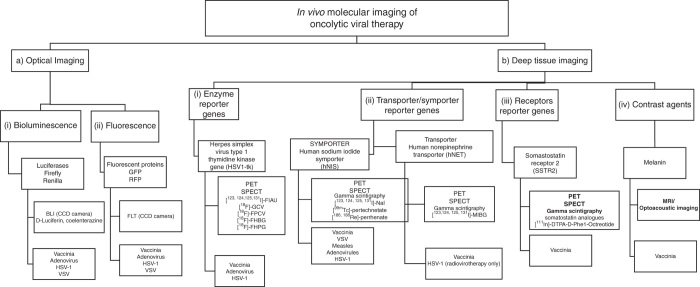
Figure 2.
Overview of current oncolytic viruses genetically encoding reporter genes utilized for in vivo molecular imaging. (A) Optical imaging: (i) genes encoding for luciferases (Firefly and Renilla) mediated bioluminescence imaging via CCD cameras utilizing D-luciferin or coelenterazine substrates; (ii) genes encoding for fluorescent proteins (GFP and RFP) mediated fluorescence imaging via CCD cameras. Viruses encoding genes mediating both optical imaging modalities so far are vaccinia, adenovirus, HSV-1 and VSV. (B) Deep tissue imaging: (i) the enzyme reporter gene HSV-1 thymidine kinase is activated by several 18F tagged substrates such as ganciclovir (18F-GCV) and may be imaged via PET and SPECT with viruses encoding this gene so far being vaccinia, HSV-1 and adenovirus; (ii) the human sodium iodide symporter hNIS is one of the most promising reporter genes investigated so far, facilitating deep tissue imaging via uptake of several species of carrier free radionuclide probes such as radioiodide, technecium, and rhenium with viruses designed to encode this symporter so far being vaccinia, VSV, measles, adenovirus, and HSV-1; the human norepinephrine transporter hNET facilitates specific uptake of radiolabeled MIBG with viruses encoding this gene so far being a vaccinia and HSV-1 strain. Both reporter genes enable imaging of viral replication via PET, SPECT, and gamma scintigraphy; (iii) the somatostatin SSRT2 receptor reporter gene facilitates uptake of somatostatin analogues usually radiolabelled with indium ([111In]-DTPA-D-Phe1-Octreotide) and may be imaged via PET, SPECT and gamma scintigraphy with viruses encoding this gene so far being only a vaccinia strain; (iv) a vaccinia virus has been genetically engineered to carry the gene encoding for melanin, which may be used as a contrast agent and facilitated imaging of viral replication via MRI and optoacoustic imaging.