Abstract
Fibromyalgia syndrome (FM) is a debilitating chronic pain condition, which afflicts primarily females. Although the etiology of this illness is not completely understood, FM pain is thought to rely on enhanced pain sensitivity maintained by central mechanisms. One of these mechanisms is central pain amplification, which is characterized by altered temporal summation of second pain (TSSP). Here we use a TSSP paradigm and functional MRI (fMRI) of the spinal cord, brainstem, and brain to noninvasively examine the central nervous system contributions to TSSP in FM patients and normal controls (NC). Functional MRI of pain‐free female adults (N = 15) and FM patients (N = 14) was conducted while brief, repetitive heat pain stimuli (0.33 Hz) were applied to the thenar eminence of the hand (C6 dermatome). The stimulus intensity was adjusted to each participant's heat pain sensitivity to achieve moderate pain. Data were analyzed by means of a General Linear Model and region‐of‐interest analyses. All participants demonstrated significant pain summation in the TSSP condition. FM subjects, however, required significantly lower stimulus intensities than NC to achieve similar TSSP. fMRI analyses of perceptually equal TSSP identified similar brain activity in NC and FM subjects; however, multiple areas in the brainstem (rostral ventromedial medulla and periaqueductal grey region) and spinal cord (dorsal horn) exhibited greater activity in NC subjects. Finally, increased after‐sensations and enhanced dorsal horn activity was demonstrated in FM patients. In conclusion, the spinal and brainstem BOLD responses to TSSP are different between NC and FM patients, which may indicate alterations to descending pain control mechanisms suggesting contributions of these mechanisms to central sensitization and pain of FM patients. Hum Brain Mapp 37:1349‐1360, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: fibromyalgia, fMRI, spinal cord, brainstem, temporal summation of pain
INTRODUCTION
Fibromyalgia syndrome (FM) is a highly prevalent chronic pain disorder, estimated to affect 2–5% of the population, afflicting primarily females [Clauw et al., 2011]. The main symptoms include diffuse pain and stiffness in the muscles or joints, accompanied by widespread hyperalgesia, general fatigue, and sleep disturbance [Clauw et al., 2011; Jensen et al., 2012]. Although the etiology of this disease is not completely understood, FM pain is believed to be maintained by both peripheral mechanisms, through tonic deep muscle inputs, and central mechanisms, by facilitation of pain processing in the spinal cord and brain [Staud, 2008; Staud et al., 2003, 2007b]. Recent brain imaging studies of FM patients have demonstrated differences in activity and connectivity within the pain inhibitory network [Jensen et al., 2009, 2012; Julien et al., 2005], changes in resting state networks [Cifre et al., 2012], and gray matter atrophy in pain‐related brain areas [Robinson et al., 2011]. Therefore, there is accumulating evidence of alterations of pain processing at the central level in FM.
One method to test increased central sensitivity is by employing temporal summation of second pain (TSSP) paradigms, which evoke a C‐fiber‐dependent enhancement (or “wind‐up”) of the dorsal horn neurons' excitability. Abnormal behavioral responses to temporal summation paradigms in patients with FM strongly suggest central pain processing abnormalities. Specifically, FM patients have shown enhanced TSSP at 0.33 Hz and TSSP at lower stimulus frequencies (0.17 Hz) [Konietzny and Hensel, 1975; Staud et al., 2008a, 2004, 2007b]. Furthermore, FM patients also displayed heightened TSSP after‐sensations and maintained a state of centrally enhanced pain sensitivity compared to normal pain‐free control (NC) subjects [Konietzny and Hensel, 1975; Staud et al., 2004, 2007b]. However, when stimulus intensities were calibrated to evoke equivalent TSSP responses, no differences in brain responses were found between FM patients and NC, indicating that enhanced TSSP mechanisms were not due to selective cortical activations [Staud et al., 2007a, 2008b]. These findings suggest that abnormal TSSP and thus central sensitization of FM patients may depend not only on spinal cord mechanisms but also on enhanced pain facilitation or decreased inhibition [Staud et al., 2008b].
Therefore, using the TSSP paradigm and fMRI of the brain, brainstem, and spinal cord, we noninvasively probed central mechanisms of the pain response in subcortical regions of patients with FM. This study builds on previous work examining TSSP in the brain of NC and FM patients [Staud et al., 2008b] and our recent work in healthy pain‐free volunteers, where we demonstrated the spinal cord and brainstem responses associated with TSSP [Bosma et al., 2015]. The aim of this study is to characterize the fMRI responses in the spinal cord and brainstem that correspond with TSSP in patients with FM, as compared to NC. We also acquired brain fMRI data to compare the results of this study with previous studies, to establish a foundation for the results presented in the spinal cord and brainstem. We hypothesize that, using pain‐sensitivity calibrate temperatures, there will be no significant differences in the TSSP‐related brain response. We also hypothesize that there will be significantly decreased fMRI responses in the spinal cord and brainstem, that reflect alterations in the descending control system.
MATERIALS AND METHODS
Participants
We recruited 20 pain‐free female participants (normal controls; NC) (age range = 21–55, M age = 39 ± 10.2) and 20 participants with FM (age range = 21–52, M age = 39 ± 4.9) from the local community or local FM support groups. Normal controls were previously described in detail elsewhere [Bosma et al., 2015]. All FM subjects were assessed with an algometer (FPK 10 pain test algometer, Wagner instruments) to see if they fulfilled the 1990 American College of Rheumatology Criteria for FM [Wolfe et al., 1990]. Current use of opioids or nonsteroidal anti‐inflammatory drugs was an exclusion criterion for the study. All premenopausal subjects were tested during the luteal phase of their menstrual cycles as determined by their menstrual history [Hapidou and Rollman, 1998]. Only 15 NC participants and 14 FM participants chose to complete all stages of the study, which involved 3 study sessions on separate days as described below. The number of participants was determined by an a priori power calculation based on the number of volumes acquired and the expected effect sizes. All procedures were performed in accordance with the Tri‐Council Policy Statement on Ethical Conduct for Research Involving Humans, and participants provided informed consent prior to the studies.
Experimental Design
For this study, we compared brain and spinal cord responses to TSSP in NC and FM participants. The experimental design was previously described in detail [Bosma et al., 2015]. Briefly, TSSP was evoked by repetitive noninjurious thermal stimuli applied to the skin overlying the thenar eminence of the right hand (corresponding to the 6th cervical dermatome) at a frequency of 0.33 Hz (TSSP condition). A TSSP control (TSSP‐C) condition was also applied with stimuli repeated at 0.17 Hz, which is insufficient to evoke TSSP in NC [Staud et al., 2006, 2001] (Fig. 1). The study procedures for each participant consisted of one session for quantitative sensory testing/mock‐fMRI training session, and two functional MRI sessions, as detailed below. We also established the participant's baseline somatic pain ratings, prior to each session, by asking them to rate their overall pain on a numerical scale (0–100, no sensation‐intolerable pain) [Vierck et al., 1997]. In the first training session, all participants were familiarized with the study procedures and completed questionnaires. In subsequent sessions, functional MRI studies of the spinal cord/brainstem and brain were conducted while the TSSP and TSSP‐C paradigms were applied in a counter‐balanced order.
Figure 1.
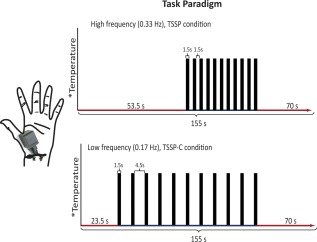
The task paradigm for the TSSP and TSSP‐C conditions. The temperature of stimulation was calibrated for each individual to produce a moderate pain rating (50 on a 100‐point scale) for the last stimulus of the TSSP paradigm. The same temperature was used for both conditions. The duration of the first baseline period was reduced for the TSSP‐C condition, so that the final baseline period was the same between conditions. For the TSSP condition, the heat stimuli were applied every 3 s, for 1.5 s, whereas in the TSSP‐C condition, the heat stimuli were applied every 6 s, also for a duration of 1.5 s. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Questionnaires
All participants were asked to complete the Beck Depression Inventory‐II (BDI‐II) [Beck et al., 1996], the State/Trait Anxiety Questionnaire [Spielberger et al., 1983], and the Pain Catastrophizing Scale [Sullivan et al., 1995]. The BDI‐II is a self‐administered 21‐item inventory which assesses the affective, motivational, cognitive, and somatic symptoms of depression. The State/Trait Anxiety Inventory consists of a 40‐item self‐report measure that is divided into two sections, with 20 items measuring the transient condition of state anxiety and 20 items devoted to the long‐standing conditions of trait anxiety. The Pain Catastrophizing Scale is a 13‐item measure consisting of descriptions of various thoughts and feelings related to pain.
Training Session
At the beginning of the first session, participants were trained to use a standardized numerical pain scale (NPS) to rate the magnitude/intensity of their heat pain experience [Staud et al., 2006; Vierck et al., 1997]. The scale ranges from 0 to 100, in increments of 5, with verbal descriptors at intervals of 10: 0 = no sensation, 10 = warm, 20 = a barely painful sensation (i.e., pain threshold), 30 = very weak pain, 40 = weak pain, 50 = moderate pain, 60 = slightly strong pain, 70 = strong pain, 80 = very strong pain, 90 = nearly intolerable pain, and 100 = intolerable pain. Participants were shown the NPS scale continuously while they rated their sensations. Prior to the onset of the heat pain conditions, the anchors of the scale were read to the participants, and they were instructed to rate their general pain sensation numerically as the baseline in order to differentiate between somatic pain and heat pain. This scale has been preferred for discriminating levels of sensation and rating a tandem series of sensations [Staud et al., 2007a; Vierck et al., 1997].
After participants were instructed on the scale, a series of threshold and calibration tests were performed. All heat sensations were applied to the skin via an MR‐compatible, Peltier thermode (Medoc®, Ramat Yishai, Israel). The Medoc device was programmed to control the temperature while the thermode was held by one of the experimenters and applied manually to the skin as needed for the specific test or stimulus. First, participants were asked to rate their pain sensations to three different temperatures (FM at 44°C, 45°C, 46°C; NC at 45°C, 46°C, 47°C), which were applied to the skin of the forearm via the thermode for 2 s. Ratings from this test served to confirm that participants could distinguish between the different temperatures and understood the rating scale. The thermode was then heated to 42°C and contact with the thenar eminence was repeated eight times, every 3 s (i.e., a frequency of 0.33 Hz). For the series of contacts, the experimenter was guided by audio cues (imperceptible to the participant) to indicate the duration (1.5 s) and timing onset of each contact. Participants were instructed to rate their pain from each contact, and also to rate the sensation on their hand at 15 and 30 s after the last contact, to provide indications of after‐sensations. This process was repeated with thermal stimuli at varying temperatures (NC at 46°C, 50°C, 44°C, and 48°C, FM at 44°C, 48°C, 42°C, and 46°C). This series of tests determined if the participant experienced temporal summation of C‐fiber‐mediated second pain and guided our calibration of the appropriate temperature required to achieve a sensitivity‐adjusted final NPS rating of 50 ± 10 units. Therefore, the optimal stimulus intensity was varied as a function of each subject's TSSP sensitivity. A rating of 50 ± 10 NPS units was chosen because healthy participants were unlikely to experience prolonged peripheral or central hypersensitivity at this temperature after repeated trials [Staud et al., 2007a].
fMRI Training
During the initial training session, all participants underwent an fMRI mock scanning session to familiarize themselves with the actual fMRI environment. Participants lay down on the MRI mock scanner bed and went through the TSSP protocol as it would be presented in the subsequent MRI sessions. They viewed a rear‐projection screen (via a mirror) on which notifications were given to the participants regarding onset of stimuli and requests for verbal pain responses. As in the earlier tests, the thermode was heated to the calibrated temperature and was applied in a series of 8 brief (1.5 s) contacts to the thenar eminence of the right hand (Fig. 1). For the TSSP condition, repetitive contacts were made at an interstimulus interval (onset to onset) of 3 s (0.33 Hz), while the TSSP‐C condition (0.17 Hz) was implemented at a 6 s interstimulus interval and was unlikely to induce TSSP. While inside the mock scanner, participants were instructed to silently rate their pain to each heat contact; however, they were asked to verbally report their ratings after the first and last heat contact upon a prompt on the screen. This was done to avoid unnecessary movements that would confound data acquisition during fMRI sessions. Participants were told to let their ratings reflect what happened throughout the condition such as whether their pain increased, decreased, or if their pain stayed the same. Finally, participants verbally reported ratings 15 and 30 s after the last heat stimulus. The whole process was repeated, with a rest interval of at least 2 min to prevent long‐term sensitization of nociceptive afferents [Price et al., 1977]. Prerecorded scanner sounds were also played in the background to familiarize the participants with the acoustic experience during the actual fMRI scanning.
TSSP Scanning Design
The stimulation paradigms used during fMRI sessions were similar to those practiced during the mock fMRI session. In both TSSP and TSSP‐C conditions, 11 heat contacts were applied, every 3 s or every 6 s, respectively (Fig. 1). The stimulation periods were preceded and followed by rest periods resulting in a total duration of 155 s for each paradigm. During fMRI sessions, participants were prompted to provide ratings one time, immediately after the last heat contact, and ratings of after‐sensations were not obtained. Again, participants viewed instructions on a rear‐projection screen (via a mirror) which notified them when a new scan was about to begin, when the application of heat stimuli would begin, and when to report their ratings into a noise‐canceling microphone. Six runs of each of the TSSP and TSSP‐C conditions were implemented in a random, counterbalanced order, and a minimum of 2 min rest was given between each run.
fMRI Data Acquisition
All image data were acquired using a 3 T whole‐body MRI system (Siemens Magnetom Trio; Siemens, Erlangen, Germany). Participants were positioned supine and were supported by foam padding as needed, to ensure comfort and minimize bulk body movement. All head, neck, and spine coils were in place, and selected as needed for each component of the imaging protocol. For the spinal cord/brainstem imaging, initial localizer images were acquired in three planes as a reference for slice positioning. Data were acquired using a phased‐array spine receiver coil, posterior neck coil, and posterior elements of a head coil, and a body coil was used for transmitting radiofrequency (RF) excitation pulses. For the brain regions, functional images were acquired in 49 axial slices oriented parallel to the anterior commissure–posterior commissure (AC–PC) line using a gradient‐echo echo‐planar imaging (EPI) sequence. A 12‐channel head coil was used for detection of the MRI signal, with a body coil for transmission of RF pulses. The image acquisition parameters included a repetition time of 3 s, an echo time of 30 ms for optimal T2*‐weighted BOLD sensitivity, a flip angle of 90°, with a 211 × 211 mm field of view, and a 64 × 64 matrix to yield voxel dimensions of 3 × 3 × 3 mm3. A total of 250 volumes were acquired for each condition.
For the spinal cord and brainstem, a half‐Fourier single‐shot fast spin‐echo (HASTE) sequence was used, with BOLD contrast. A 3D volume that spanned from the T1 vertebra to above the thalamus was imaged repeatedly to produce each fMRI time‐series. Nine sagittal slices were acquired contiguously with a repetition time (TR) of 0.75 s/slice, an echo time of 76 ms to optimize the T2‐weighted BOLD sensitivity, and a 28 × 21 cm field‐of‐view with 1.5 × 1.5 × 2 mm3 resolution. A total of 138 volumes were acquired for each condition. The image quality was enhanced by means of spatial suppression pulses anterior to the spine to reduce motion artefacts caused by breathing, swallowing, and so on, and motion compensating gradients in the head–foot direction.
DATA ANALYSIS
Data Preprocessing
The brain data were preprocessed using the Statistical Parametric Mapping SPM8 package (Wellcome Institute of Cognitive Neurology, London, UK). Data were motion and slice‐time corrected, spatially normalized to the MNI template, and smoothed using a 6 mm3 full‐width at half‐maximum Gaussian kernel. The 3D spinal cord/brainstem functional imaging data were analyzed with custom‐made software written in MatLab®. Image data were first converted to NIfTI format, and were coregistered to correct for bulk motion using the nonrigid 3D registration tool in the Medical Image Registration Toolbox (MIRT) [Myronenko and Song, 2009, 2010]. The images were then resized to 1 mm3 voxels and spatially normalized using custom‐made automated normalization software written in MatLab. For the normalization, predefined sections of our normalized template, which we have generated from images of 356 participants, were matched in position and rotation angle to sections of the original image data, based on the maximum cross‐correlation. The first section identified included the corpus callosum and thalamus because these regions have distinct features that tend to make their location unambiguous. In subsequent sections, the position and the angle were weighted toward predicted values based on prior segments resulting in a stable mapping process. The mapping to the normalized template was also fine‐tuned using the MIRT toolbox[Myronenko and Song, 2009, 2010]. The normalized data were then smoothed with a 3 × 3 × 5 mm (R/L × A/P × S/I) boxcar kernel. The time‐series data from repeated acquisitions from each participant, in each study condition, were averaged prior to data analysis. This process has been shown to reduce effects of random and physiological noise, which are uncorrelated across repeated acquisitions while reinforcing BOLD responses that are time‐locked to the stimulation paradigm [Bosma and Stroman, 2014a; Stroman et al., 2012].
General Linear Model Analysis
A first‐level analysis was conducted on the preprocessed data for each participant, for each condition, using a General Linear Model (GLM) as implemented in the Statistical Parametric Mapping (SPM) software package [Worsley and Friston, 1995]. The basis set used for the GLM analysis included (1) a paradigm matching the timing of each heat contact and pre‐ and post‐ and interstimulus baseline periods (to model peripheral input to the spinal cord), (2) a term that estimated the timing of the after‐sensations of the pain response as a block paradigm, and for the spinal cord and brainstem data the GLM also included (3) two nuisance regressor terms to account for physiological noise, and 4) a constant function. The nuisance regressor terms were taken as the first two principal components obtained from a Principal Components Analysis of the time‐series data across all voxels in the spinal cord and brainstem. These terms have been shown to account for sources of global variance that are common across a large number of voxels and originate from physiological motion, whereas BOLD responses are more highly localized [Bosma and Stroman, 2014a]. The models of peripheral stimulation and after‐sensation timing were convolved with the BOLD hemodynamic response function [Worsley and Friston, 1995].
Second‐level analyses were carried out using a random‐effects method to determine the consistency of BOLD response magnitudes (beta‐values) determined from the GLM, on a voxel‐by‐voxel basis. The BOLD responses were contrasted between study conditions (TSSP vs TSSP‐C). For the brain, significant activity was inferred at a family wise error corrected p < 0.05, whereas for the spinal cord and brainstem data, significant activity was inferred at p < 0.001 uncorrected and the problem of multiple comparisons was addressed by limiting active regions to those with a spatial extent of at least 10 mm3, based on the “stat_threshold” function written by K. J. Worseley [Bosma and Stroman, 2014a, 2014b]. This difference in statistical threshold selection is to account for the difference in the number of voxels (small number in spinal cord, large number in the brain) as conventional methods employed to control for multiple comparisons are overly conservative for spinal cord images [Brooks et al., 2008; Cohen‐Adad et al., 2009; Moffitt et al., 2005].
Anatomical Region Mask
An anatomical mask was defined within the normalized template for multiple brainstem and spinal cord regions based on anatomical atlases and published descriptions, with 3D regions labelled manually on our normalized template image (used in the normalization step) [Naidich et al., 2009; Talairach and Tournoux, 1988; Williams et al., 1995]. The spinal cord segments were identified based on the distance in millimeters from the pontomedullary junction (PMJ) as described by Lang and Bartram [Lang, 1993; Lang and Bartram, 1982]. All anatomical regions described in the analyses and results below are in reference to this region mask and are expected to be in close proximity to the regions that they depict, within the accuracy of our image data and published descriptions of the anatomy.
Dorsal Horn ROI Analysis
Region‐of‐interest (ROI) analysis in the dorsal horn was carried out by defining 3D volumes in the normalized representation of the cord, as detailed below, and determining average responses over these volumes. Anatomical regions were as defined in the region mask described above. In this mask, the 6th cervical (C6) segment spans from 78 to 92 mm along the cord from the PMJ. Within each segment, the cord cross‐sectional area was divided right/left and dorsal/ventral to define quadrants. The time‐series data were extracted from the C6 right dorsal region in both the TSSP and TSSP‐C conditions, for both groups, and the average BOLD signal changes in the DH during the stimulation periods were compared.
RESULTS
Somatic Pain Ratings and Questionnaires
NC participants reported no somatic pain before the fMRI scans. In contrast, the FM subjects reported an overall NPS pain rating of M = 48, SD = 23. The mean (SD) Beck's Depression Inventory score was M = 8.8 (5) and M = 20 (13), for NC and FM subjects, respectively. The mean (SD) Spielberger State and Trait Anxiety scores for NC were M = 33 (8) and M = 38 (10), respectively, while the mean (SD) Spielberger State and Trait Anxiety scores for FM subjects were M = 47 (12) and M = 47 (11), respectively. The pain catastrophizing scores for FM were M = 25 (10) and M = 15 (13) for controls. Independent t‐tests of BDI scores indicated that the depression scores for FM patients were significantly higher compared to NC participants [t(25) = 2.9, p = 0.007]. Similarly, there was a significant difference between the NC and the FM subjects for the State and the Trait anxiety scores, [t(25)state = 3.0, p = 0.006, t(25)trait = 2.1, p = 0.04]. Finally, pain catastrophizing scores were also significantly higher for FM compared to NC subjects, [t(25) = 3.1, p = 0.005].
Behavioral Results
In the training and fMRI sessions, the heat stimulus intensity was calibrated for each individual to elicit a moderate heat pain rating after the last heat contact in the TSSP condition. The temperatures used in the NC group were significantly higher than the FM group [t(27) = 2.79, p = 0.009].
During the scanning session, pain ratings for the first and last pain stimuli were obtained immediately after the last heat stimulus. A mixed model ANOVA with between‐group (FM vs NC) and within‐group (TSSP vs TSSP‐C) variables was conducted to compare pain ratings across variables (Fig. 2 and Table 1). The ratings provided by FM participants were not significantly different from the ratings reported by NC participants [F(1,27) = 0.643, p = 0.43], corresponding with the heat stimuli being calibrated for each participant. However, comparisons of study conditions showed that the ratings in the TSSP condition were significantly different than the ratings in the TSSP‐C condition [F(1,27) = 79.29, p < 0.001]. Pain ratings to the first heat stimulus were also significantly different from those for the last stimulus [(F(1,27) = 109.22, p < 0.001)].
Figure 2.
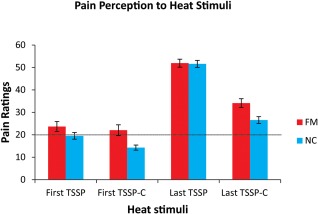
The mean pain ratings across participants for the first and last heat pain stimulus in the two conditions. In the fMRI session, all participants were asked to remember the number ratings of the pain intensity related to the first and last heat stimuli (11 heat stimuli in total) and reported their ratings at the end of the stimulation period. In both the NC and FM participants, the rating to the last heat stimulus was significantly greater than the rating to the first (p < 0.001). The ratings to the last heat stimulus was also significantly different between conditions (TSSP > TSSP‐C) (p < 0.001). A rating of 20 represents the pain threshold (dotted line). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Pain ratings for each group in each condition
| Condition | FM rating, mean (SD) | NC rating, mean (SD) |
|---|---|---|
| TSSP first stimuli | 23.7 ± 19.68 | 19.52 ± 14.33 |
| TSSP last stimuli | 51.91 ± 15.80 | 51.59 ±14.61 |
| TSSP‐C first stimuli | 22.04± 21.41 | 14.30 ± 10.72 |
| TSSP‐C last stimuli | 34.11 ± 17.34 | 26.56 ±14.61 |
| After‐sensations TSSP | 13.86 ± 4.8 | 9.17 ± 5.35 |
| After‐sensations TSSP‐C | 11.12 ± 6.32 | 4.55 ± 4.34 |
Dependent sample t‐tests were conducted to further investigate the differences between the TSSP and TSSP‐C conditions. Reported pain ratings were significantly higher for the last stimulus compared to the first stimulus in the TSSP condition, in both the NC and the FM participant groups [NC; t(14) = 9.7, p < 0.001, FM; t(13) = 6.58, p < 0.001]. The pain ratings were also significantly higher for the last stimulus in the TSSP condition compared to the TSSP‐C condition [NC; t(14) = 7.18, p < 0.001, FM; t(13) = 5.54, p < 0.001]. Importantly, there were no significant differences between the ratings in either condition between the training session and the fMRI sessions (all p > 0.05).
After‐sensations were compared separately from the pain caused by the stimulus. The ratings reported at 15 and 30 s after the last stimulus were averaged and were compared between and within groups (Fig. 3). The after‐sensation pain reported by the FM group was significantly higher than for the NC group [F(1,21) = 10.17, p = 0.004]. However, within the FM group, the after‐sensations for the TSSP and TSSP‐C conditions was not significantly different, as determined by a dependent sample t‐test [t(12) = 1.77, p = 0.10].
Figure 3.
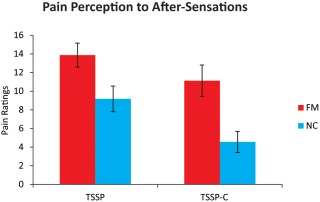
Reported ratings of after‐sensations obtained from each participant, for each condition, during the fMRI training sessions. Ratings of residual sensations/pain on the hand were obtained 15 and 30 s after the last heat stimulus and were averaged for each participant. The ratings of the FM participants were significantly higher (FM > NC) (p < 0.05). In the FM group, there was no significant difference between the ratings in the TSSP condition compared to the TSSP‐C condition. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
BOLD Activity Associated with TSSP
TSSP vs TSSP‐C in FM
Results of the TSSP‐related brain response revealed no significant differences between the FM and NC groups. As the brain data were only acquired to compare to previous findings, no further analyses or results are reported here [Staud et al., 2007a, 2008b]. In the spinal cord and brainstem, a GLM analysis was performed on data from both the TSSP and TSSP‐C conditions and revealed active voxels in a number of pain‐related regions. For the FM groups, a contrast analysis was performed to determine areas of the cord and brainstem responding more to the TSSP paradigm, compared to the TSSP‐C condition (Fig. 4). Detailed comparisons between the conditions for the NC group are described elsewhere [Bosma et al., 2015]. Very few regions demonstrated different task‐related activity. Only small areas in the region near the nucleus tractus solitarius (NTS) and near the rostral ventromedial medulla (RVM) were significantly different between the two conditions. There were no areas that had greater activity in the TSSP‐C condition compared to the TSSP condition.
Figure 4.
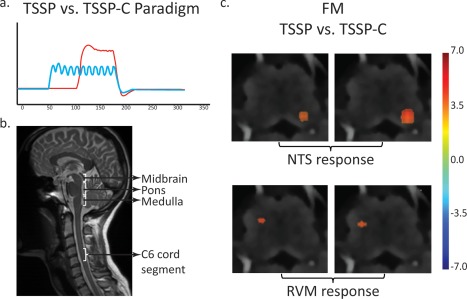
Results of the contrast analysis comparing BOLD signal changes between the TSSP and TSSP‐C conditions in the FM group. (a) On the left is an illustration of the task paradigms convolved with the hemodynamic response function as used in the GLM analysis (red = TSSP paradigm, blue = TSSP‐C). (b) A midline sagittal slice from the functional data of one participant is shown for reference and illustrates the approximate location of the midbrain, pons, medulla, and C6 cord segment. (c) Key areas with significantly different responses to the stimulation paradigm are demonstrated in the vicinity of the NTS and RVM in the medulla. The results are overlaid on high resolution transverse slices. The color scale indicates the significance of areas with different responses between the conditions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Dorsal horn ROI analysis
In the NC group, the time course average BOLD signal change in the DH during the stimulation period was significantly greater in the TSSP condition compared to the TSSP‐C condition. However, in the FM group, there was no significant difference between the percent signal change during heat pain stimulation in the TSSP vs TSSP‐C condition (Fig. 5).
Figure 5.
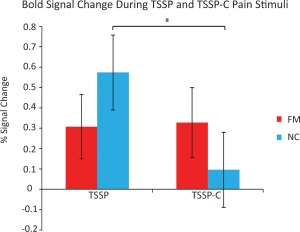
The % signal change from the C6 dorsal horn during task stimulation in each condition (TSSP vs TSSP‐C) and each group (FM and NC). Error bars indicate standard error, p < 0.05. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Contrast between FM and NC
Contrasts between the FM and NC groups revealed differing responses in key areas involved in the descending modulation of pain. In the TSSP condition, differences in task‐related activity (i.e., BOLD responses) were observed in anatomical proximity to the dorsal and ventral horns (DH and VH), the RVM, the NTS, and in the locus coeruleus (LC). There are also several regions that had greater responses in the TSSP‐C condition including the DH, VH, NTS, LC, and the periaqueductal grey region (PAG). There were no regions that had greater activity in the FM participants (Fig. 6).
Figure 6.
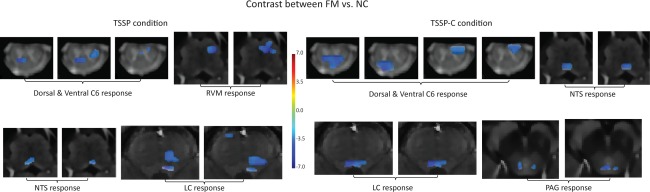
Results of the contrast analysis comparing BOLD signal changes between the FM and NC groups in both the TSSP and TSSP‐C conditions. On the left is the contrast between groups for the TSSP condition. Key areas with significantly different responses to the stimulation paradigm are demonstrated in the vicinity of the dorsal and ventral horns, RVM and NTS in the medulla, and LC in the pons. Contrast of the TSSP‐C condition between groups (right) reveals differences in the dorsal and ventral horns, NTS in the medulla, LC in the pons, and PAG in the midbrain. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The responses to the after‐sensations were also identified and compared between groups (Fig. 7). There was significantly greater BOLD activity in the ipsilateral dorsal horn in the FM group compared to the NC group.
Figure 7.
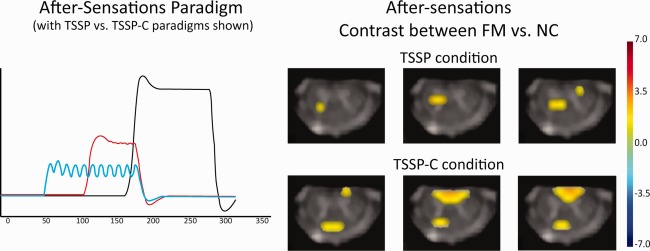
Key areas with significantly different responses to the after‐sensations between FM and NC are shown to be the right dorsal horn region of the C6 segment in both the TSSP and TSSP‐C conditions. On the left is an illustration of the after‐sensation paradigms convolved with the hemodynamic response function as used in the GLM analysis (red = TSSP paradigm, blue = TSSP‐C). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
This is the first study to use fMRI to investigate the neural mechanisms of TSSP in the spinal cord and brain of women with FM and compare them to NC. Temperatures used for repetitive heat stimuli were calibrated to produce similar TSSP for FM patients and NC when presented at fast frequencies, but when the same stimuli were presented at low frequencies, the FM patients continued to demonstrate pain summation. Furthermore, there were significant TSSP after‐sensations in FM but not in NC subjects. Together, these findings suggest the presence of central sensitization in FM patients. Our study identified several neural correlates of TSSP in the FM and NC groups. First, in contrast to NC, there were only minor differences in BOLD responses of FM patients in the TSSP and TSSP‐C conditions. This agrees with previous findings that temporal summation for FM subjects occurred at substantially lower frequencies of stimulation [Staud et al., 2003, 2001]. Second, BOLD responses related to TSSP after‐sensations of FM patients in the spinal cord were significantly greater than NC. Furthermore, there was significantly increased activity in the spinal cord and brainstem areas of NC during TSSP compared to FM patients suggesting abnormal descending modulation of pain in FM. Additional differences in NTS and RVM BOLD responses of FM patients but not NC between TSSP and TSSP‐C conditions provided further evidence for abnormalities of spinal cord pain processing in FM. Our recent findings in healthy control (NC) participants identified enhanced activity in the spinal cord dorsal horn at C6 and in multiple areas of the brainstem, in response to the TSSP condition [Bosma et al., 2015]. The results in FM participants in this study, therefore, provide evidence of altered responses to repeated stimuli.
Abnormal Spinal Pain Processing in FM
Enhanced ratings of after‐sensations, which indicate heightened sensitivity, occurred in both conditions for FM patients (Fig. 3 and Table 1). This provides further evidence of altered spinal cord pain processing in the TSSP‐C condition. These behavioral findings are consistent with previous reports of enhanced maintenance and after‐sensations in FM subjects. Specifically, enhanced second pain in FM subjects could be maintained for up to 120 s, using stimuli delivered at 0.16 and 0.08 Hz which do not evoke or maintain TSSP in NC subjects [Staud et al., 2004]. In addition, FM subjects also report after‐sensation of greater intensity which are longer lasting [Staud et al., 2001]. Our fMRI results parallel these behavioral findings as the BOLD signals were sustained after the final heat stimulus with greater dorsal horn activity in the FM group, compared to NC. Despite the potential physiological significance of characterizing the neural underpinnings of after‐sensations, we acknowledge that our study did not fully explore this aspect of the TSSP response, and ratings of after‐sensations were only acquired in the training session and were calculated as an average of ratings from 15 and 30 s after the final pain stimulus. Perhaps as a result, the average after‐sensations were not in the pain range for most subjects. Future study designs can be optimized to increase the number of ratings of the after‐sensations, and include the acquisition of these rating in the fMRI session. Nevertheless, these findings build on our other results and reconfirm the role of abnormal central mechanisms in FM.
We calibrated the stimulus intensity for each participant to evoke a comparable level of pain to ensure that the sensory‐discriminatory aspects of nociception did not differ. Consistent with previous reports, we found that the temperatures required to evoke similar TSSP were significantly lower in the FM group compared to the NC group [Cook et al., 2004; Gracely et al., 2002; Petzke et al., 2003; Staud et al., 2008b]. In general, when pain stimulus intensities are calibrated to the same level, no differences in brain fMRI responses are expected, based on prior studies [Cook et al., 2004; Gracely et al., 2002; Staud et al., 2008b]. Consistent with this expectation, and with Staud et al., who carried out a detailed analysis of TSSP‐related brain responses between FM and NC subjects [Staud et al., 2008b] we found no significant differences in BOLD responses between the groups. Interesting however, Cheng et al. (2015) have recently demonstrated in healthy controls that greater TSSP is positively associated with functional connectivity in ascending pain pathways (thalamus and BA 3a), and negatively associated with connectivity in a descending pain pathway (RVM and sgACC). Investigation of ascending and descending pain pathway responses to TSSP variability in chronic pain patients is of great interest for future studies.
Abnormal Pain Modulation in FM
Although we found no differences in brain regions in response to pain‐sensitivity calibrated temperatures, we demonstrate significant differences in a number of brainstem and spinal cord regions between FM and NC groups. Comparisons of the two groups demonstrate greater fMRI responses in the NC group in both TSSP and TSSP‐C conditions (Fig. 6), including the dorsal and ventral horn (C6), the RVM, NTS, LC, and the PAG. These regions in the brainstem are known to modulate dorsal horn responses and play an important role in the descending modulation of pain [Millan, 2002]. Normal pain processing is a dynamic balance of pain inhibition and facilitation and dysregulation of either of these mechanisms may contribute to the development and maintenance of chronic pain. Our findings suggest that the descending control mechanisms are altered in FM patients as demonstrated by altered brainstem and spinal cord responses to TSSP. These results are consistent with previous studies showing FM specific decreases in activity in the rostral anterior cingulate cortex and areas in the brainstem involved in pain inhibition [Jensen et al., 2009] and with reports of altered functional connectivity in pain networks during tasks [Burgmer et al., 2012] and in the resting state [Cifre et al., 2012].
LIMITATIONS
There are several factors that could contribute to the differences we observed between FM and NC groups including differences in pain anticipation, the contribution of spontaneous pain signals arising in chronic pain conditions, and other psychological factors. Previous studies have demonstrated that the anticipation of pain results in different baseline fMRI response preceding experimental pain [Burgmer et al., 2010; Hsieh et al., 1999; Ploghaus et al., 1999]. For example, Ploghaus et al. (1999) found increases in the ACC, medial PFC, and the anterior insula during the anticipation of thermal [Ploghaus et al., 1999]. These anticipation‐related fMRI responses have been shown to be different in FM participants compared to healthy control participants [Burgmer et al., 2010]. Recent work in the spinal cord has also revealed that pain anticipation modulates fMRI responses at the level of the spinal cord dorsal horn [Geuter and Buchel, 2013]. Therefore, the minimal differences between conditions (TSSP vs TSSP‐C) in the FM group and the substantial differences between the NC and FM groups that we report may be influenced by pain‐anticipation responses. These responses occur before the onset of the stimulus and could alter the spinal cord and brainstem response to the stimulus‐evoked pain. As a result, these responses could reduce the fit of the time‐series data to the model of the timing of the heat pain paradigm, and could, in part, account for the difference between groups. Future studies, which examine the spatiotemporal dynamics of the pain‐anticipatory responses, are required.
Chronic pain patients experience ongoing spontaneous pain, which has been demonstrated to involve specific neuronal mechanisms, distinct from the brain hemodynamic responses observed in acute experimental pain [Baliki et al., 2006]. BOLD responses to spontaneous pain may result in a potential hemodynamic ceiling effect that would limit the hemodynamic brain responses to experimental pain stimuli [Davis et al., 2012]. Currently, the interactions between the hemodynamic brain response to spontaneous pain and acute stimulus‐evoked pain are not well understood. Our TSSP‐related results in the FM group are likely confounded by hemodynamic brain responses to spontaneous pain.
FM is not a homogeneous syndrome but varies in the comorbidity of anxiety and depression [Thieme et al., 2004]. Therefore, these psychological factors could also be contributing to the differences in TSSP‐related responses observed between groups. Importantly, other potential confounds were minimized in regards to analgesic medications (no current NSAIDs or Opioid), stage of menstrual cycle, and other factors influencing pain processing (e.g., gender). Given the range of depression and anxiety scores, pain sensitivity, and fMRI results, further investigation is warranted to determine the relationship between these factors and altered spinal cord pain processing.
One limitation of spinal cord and brainstem imaging is the lack of a standard, validated anatomical MRI template with the structures of the brainstem clearly outlined that can be co‐registered to functional data. We have created our own normalized template using anatomical references to identify the structures [Naidich et al., 2009] and have used this template as a reference to identify the areas of activity. However, we recognize that these voxels are only in proximity to the anatomical structures of interest and that our spatial resolution to delineate small brainstem nuclei is limited. Finally, consistent with previous spinal cord imaging studies, we used an uncorrected P value to evaluate the statistical inference [Brooks et al., 2008; Moffitt et al., 2005] and a cluster thresholding procedure to control for multiple comparisons [Bosma et al., 2015].
CONCLUSIONS
Our study is the first to use fMRI to characterize pain processing in the spinal cord and brainstem of FM patients and to demonstrate abnormal TSSP in FM. Our results indicate abnormal activity in regions of the spinal cord (DH) and brainstem (RVM, NTS, LC, and PAG) which suggest dysfunction in the descending control of pain. We also demonstrate increased hemodynamic responses in the dorsal horn of the spinal cord during painful TSSP aftersensations of FM patients. Altogether, our results provide strong evidence for altered cerebral–midbrain–spinal mechanisms of TSSP which may be relevant for FM pain.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
Dr Staud's effort was partially funded by NIH. We would also like to thank Don Brien for his help with acquiring the data, and Janet Mirtle‐Stroman for her help with participant recruitment. All authors confirm that they have no conflicts‐of‐interest to declare.
REFERENCES
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV (2006): Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26:12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W (1996): Comparison of Beck Depression Inventories ‐IA and ‐II in psychiatric outpatients. J Person Assess 67:588–597. [DOI] [PubMed] [Google Scholar]
- Bosma, RL , Ameli Mojarad, E , Leung, L , Pukall, C , Staud, R , Stroman, PW (2015): Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Hum Brain Mapp 36:5038–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma RL, Stroman PW (2014a): Assessment of data acquisition parameters, and analysis techniques for noise reduction in spinal cord fMRI data. Magn Reson Imaging 32:473–481. [DOI] [PubMed] [Google Scholar]
- Bosma, RL , Stroman, PW (2014b): Spinal cord response to stepwise and block presentation of thermal stimuli: A functional MRI study. J Magn Reson Imag JMRI 1318–1325. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Beckmann CF, Miller KL, Wise RG, Porro CA, Tracey I, Jenkinson M (2008): Physiological noise modelling for spinal functional magnetic resonance imaging studies. Neuroimage 39:680–692. [DOI] [PubMed] [Google Scholar]
- Burgmer M, Pfleiderer B, Maihoefner C, Gaubitz M, Wessolleck E, Heuft G, Pogatzki‐Zahn E (2012): Cerebral mechanisms of experimental hyperalgesia in fibromyalgia. Eur J Pain 16:636–647. [DOI] [PubMed] [Google Scholar]
- Burgmer M, Pogatzki‐Zahn E, Gaubitz M, Stuber C, Wessoleck E, Heuft G, Pfleiderer B (2010): Fibromyalgia unique temporal brain activation during experimental pain: a controlled fMRI Study. J Neural Transm 117:123–131. [DOI] [PubMed] [Google Scholar]
- Cheng JC, Erpelding N, Kucyi A, DeSouza DD, Davis KD (2015): Individual differences in temporal summation of pain reflect pronociceptive and antinociceptive brain structure and function. J Neurosci 35:9689–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez‐Roldan A, Martinez‐Jauand M, Birbaumer N, Chialvo DR, Montoya P (2012): Disrupted functional connectivity of the pain network in fibromyalgia. Psychosomatic Med 74:55–62. [DOI] [PubMed] [Google Scholar]
- Clauw DJ, Arnold LM, McCarberg BH (2011): The science of fibromyalgia. Mayo Clinic Proceedings 86:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen‐Adad J, Hoge RD, Leblond H, Xie G, Beaudoin G, Song AW, Krueger G, Doyon J, Benali H, Rossignol S (2009): Investigations on spinal cord fMRI of cats under ketamine. Neuroimage 44:328–339. [DOI] [PubMed] [Google Scholar]
- Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH (2004): Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol 31:364–378. [PubMed] [Google Scholar]
- Davis KD, Racine E, Collett B (2012): Neuroethical issues related to the use of brain imaging: can we and should we use brain imaging as a biomarker to diagnose chronic pain? Pain 153:1555–1559. [DOI] [PubMed] [Google Scholar]
- Geuter S, Buchel C (2013): Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci 33:13784–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ (2002): Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthr Rheum 46:1333–1343. [DOI] [PubMed] [Google Scholar]
- Hapidou EG, Rollman GB (1998): Menstrual cycle modulation of tender points. Pain 77:151–161. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stone‐Elander S, Ingvar M (1999): Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci Lett 262:61–64. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SCR, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M (2009): Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 144:95–100. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J (2012): Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain 8:32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien N, Goffaux P, Arsenault P, Marchand S (2005): Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 114:295–302. [DOI] [PubMed] [Google Scholar]
- Konietzny F, Hensel H (1975): Letters and notes: Warm fiber activity in human skin nerves. Pflugers Archiv: Eur J Physiol 359:265–267. [DOI] [PubMed] [Google Scholar]
- Lang, J. (1993) Clinical Anatomy of the Cervical Spine. New York: Thieme Medical Publishers; 192 p. [Google Scholar]
- Lang J, Bartram CT (1982): Fila radicularia of the ventral and dorsal radices of the human spinal cord. Gegenbaurs Morphol Jahrb 128:417–462. [PubMed] [Google Scholar]
- Millan MJ (2002): Descending control of pain. Progr Neurobiol 66:355–474. [DOI] [PubMed] [Google Scholar]
- Moffitt MA, Dale BM, Duerk JL, Grill WM (2005): Functional magnetic resonance imaging of the human lumbar spinal cord. J Magn Reson Imag JMRI 21:527–535. [DOI] [PubMed] [Google Scholar]
- Myronenko, A , Song, XB (2009): Image registration by minimization of residual complexity. Proc Cvpr Ieee 49–56. [Google Scholar]
- Myronenko A, Song XB (2010): Intensity‐based image registration by minimizing residual complexity. IEEE T Med Imaging 29:1882–1891. [DOI] [PubMed] [Google Scholar]
- Naidich TPDH, Delman BN, Sorensen AG, Kollias SS, Haacke EM (2009): Internal Architecture of the Brain Stem with Key Axial Sections. Duvernoy's Atlas of the Human Brain Stem and Cerebellum. New York: Springer‐Verlag/Wien; pp 79–82. [Google Scholar]
- Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH (2003): Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain 105:403–413. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN (1999): Dissociating pain from its anticipation in the human brain. Science 284:1979–1981. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH (1977): Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain 3:57–68. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R (2011): Gray matter volumes of pain‐related brain areas are decreased in fibromyalgia syndrome. J Pain 12:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983): Manual for the State‐Trait Anxiety Inventory (STAI) (Self Evaluation Questionnaire). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Staud R (2008): The role of peripheral input for chronic pain syndromes like fibromyalgia syndrome. J Musculoskelet Pain 16:67–74. [Google Scholar]
- Staud R, Bovee CE, Robinson ME, Price DD (2008a): Cutaneous C‐fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain 139:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJJ (2003): Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 102:87–95. [DOI] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD (2008b): Brain activity associated with slow temporal summation of C‐fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain 12:1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD (2007a): Brain activity related to temporal summation of C‐fiber evoked pain. Pain 129:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Price DD, Fillingim RB (2006): Advanced continuous‐contact heat pulse design for efficient temporal summation of second pain (windup). J Pain 7:575–582. [DOI] [PubMed] [Google Scholar]
- Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ (2004): Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain 110:689–696. [DOI] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Price DD (2007b): Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain 8:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD (2001): Abnormal sensitization and temporal summation of second pain (wind‐up) in patients with fibromyalgia syndrome. Pain 91:165–175. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Bosma RL, Kornelsen J, Lawrence‐Dewar J, Wheeler‐Kingshott C, Cadotte D, Fehlings MG (2012): Advanced MR imaging techniques and characterization of residual anatomy. Clin Neurol Neurosurg 114:460–470. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, Pivik J (1995): The pain catastrophizing scale: Development and validation. Psychol Assess 7:524–532. [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐Planar Sterotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers Inc. [Google Scholar]
- Thieme K, Turk DC, Flor H (2004): Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosomatic Med 66:837–844. [DOI] [PubMed] [Google Scholar]
- Vierck CJJ, Cannon RL, Fry G, Maixner W, Whitsel BL (1997): Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol 78:992–1002. [DOI] [PubMed] [Google Scholar]
- Williams PLBL, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ (1995): Gray's Anatomy: The Anatomical Basis of Medicine and Surgery. New York: Churchill‐Livingstone; p 975–1011. [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. (1990): The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism 33:160–172. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited–again. Neuroimage 2:173–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


