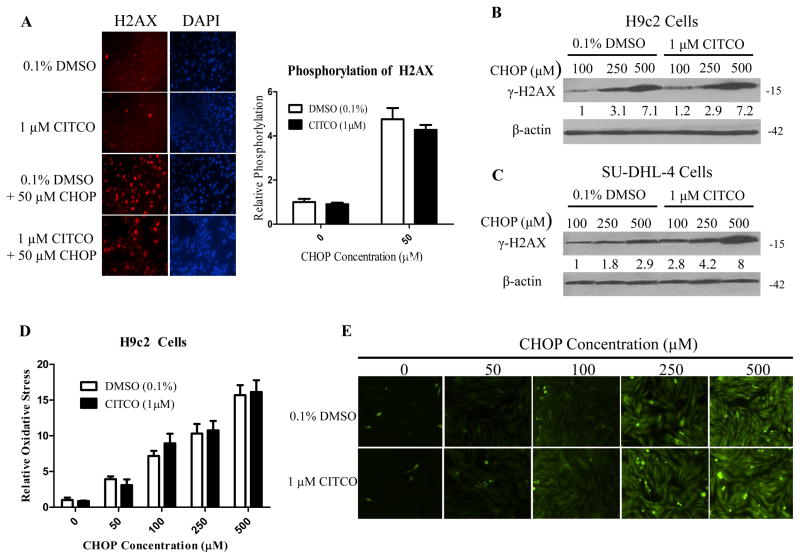
Figure 5. CITCO didn’t enhance CHOP-induced H2AX phosphorylation and oxidative stress in H9c2 cells.
HPH/SU-DHL-4/H9c2 co-culture was treated with 0.1% DMSO, CITCO (1 μM), 0.1% DMSO/CHOP, or CITCO/CHOP at indicated concentrations for 24 h. H9c2 cells removed from the co-culture system were fixed with 4% paraformaldehyde, then immune-stained and imaged for phosphorylation at the serine-139 position of histone H2AX as detailed in the Materials and Methods. Relative H2AX phosphorylation was quantified using ImageJ Software from the National Institutes of Health and normalized to vehicle control (A). In separate experiments, western blot analysis was performed in H9c2 (B) and SU-DHL-4 (C) cells isolated from co-cultures following 24 h treatment of CHOP in the presence of absence of CITCO (1 μM). To assess oxidative stress in H9c2 cell under the same treatments, H9c2 cells were incubated with 10 μM 2’,7′-dichlorofluorescein diacetate (DFDA) at 37°C and 5% CO2 for 40 min. The oxidation of DFDA to the fluorescent dichlorofluorescein was quantified using NIH ImageJ Software (D). Representative images via fluorescence microscopy were shown in (E).