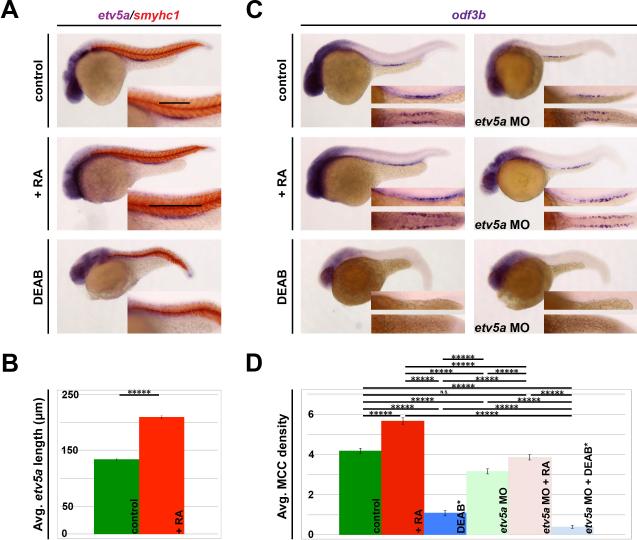
Figure 5. RA signaling acts upstream of etv5a to promote MCC fate.
(A) WISH analysis on 24 hpf embryos demonstrates an increased etv5a expression domain after treatment with exogenous retinoic acid (+RA). Conversely, treatment with the pan-RA inhibitor DEAB completely ablates etv5a expression in the pronephros. Insets show a magnified view of the pronephros, where black bars denote the etv5a domain. (B) Exogenous RA significantly increased average etv5a length (μm) in the pronephros, but etv5a expression is lost after DEAB treatment. (C) Exogenous RA increases odf3b expression in both control and etv5a morphant embryos (etv5a MO), however etv5a morphants still appear to have a reduction of odf3b transcripts when compared to control siblings analyzed by WISH. odf3b expression is greatly reduced in both control and etv5a morphant embryos treated with DEAB. A magnified lateral (top) and dorsal (bottom) view of the odf3b domain is presented in the insets. (D) Quantification shows a significant increase of average MCC density/somite in etv5a morphants + RA compared to etv5a morphants, and that treatment with RA rescues average MCC density in etv5a morphants to the control value. Morphants treated with DEAB have a significantly lower MCC density/nephron* than control embryos treated with DEAB. At least 50 embryos were analyzed for each treatment, and the error bars represent standard error. P-values: *****p<0.001