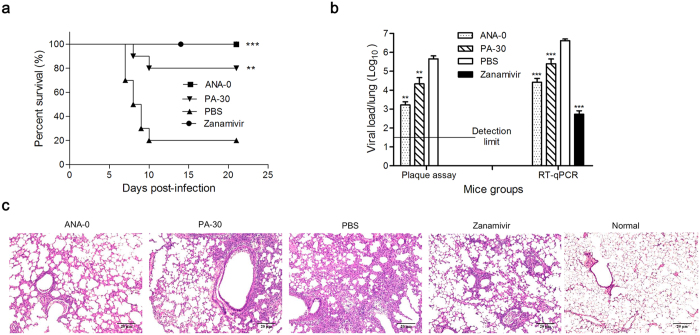
Figure 5. In vivo antiviral activity of ANA-0 and PA-30.
(a) Mice (10 per group) infected with LD80 (500 PFU/mouse) of mouse-adapted A/HK/415742Md/09 H1N1 virus were treated with 2 mg/kg/day of ANA-0 or PA-30 or zanamivir or PBS by intranasal administration. Treatments started at 6 h after virus challenge and continued for 6 doses in 3 days (2 doses/day). Difference between groups were compared and analyzed using Log-rank (Mantel-Cox) test. ***indicates p < 0.001 and **indicates p < 0.01 as compared to PBS-treated group. (b) Four mice from each group were euthanized at day 4 post-infection and lungs were collected for detection of viral loads by plaque assay (detection limit: 1:50) and RT-qPCR. The plaque was undetectable in the lung samples of zanamivir-treated mice. The results are presented as the mean values + SD. Differences between groups were compared and analyzed using a one-way ANOVA. ***indicates p < 0.001 and **indicates p < 0.01 as compared to PBS-treated group. (c) Histopathologic changes in mouse lung tissues collected at day 4 post-infection. Representative histologic sections of the lung tissues from the mice were stained with H&E (magnification: × 100). Less inflammatory infiltrate and thickening of the alveolar septum (as alveolar damage) are shown in samples from mice treated with ANA-0, PA-30 and zanamivir as compared to that from PBS-treated mice.