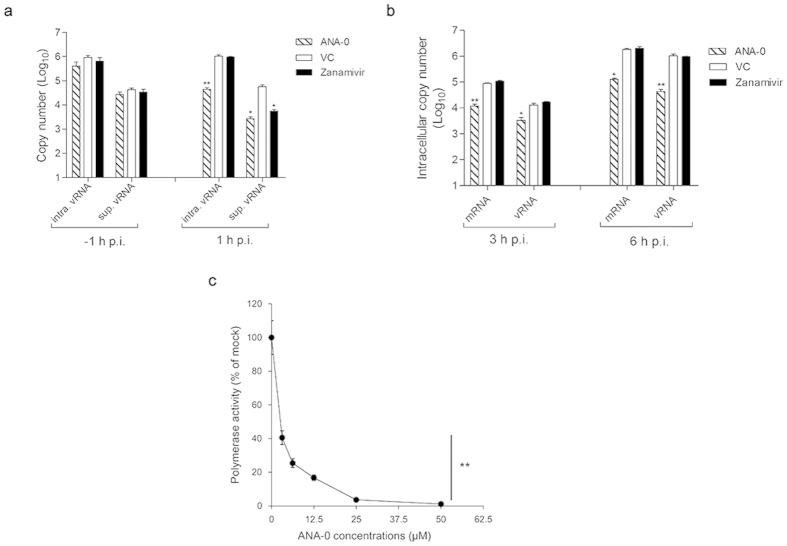
Figure 6. Antiviral mechanism of ANA-0.
(a) Intracellular viral RNA (intra. vRNA) and supernatant viral RNA (sup. vRNA) were quantified in a time-of-addition assay. MDCK cells were inoculated with influenza H1N1 virus (MOI = 2), while ANA-0 (20 μM) or zanamivir (100 μM) was added at the time of virus absorption (−1 h) and then removed or at 1 h post-infection (p.i.) and then maintained in the medium. vRNA copies in the cells or supernatants were determined at 6 h p.i. (b) MDCK cells were infected with influenza H1N1 virus with MOI of 2 for 1 h. The cells were washed and maintained in the medium containing ANA-0 (20 μM), or zanamivir (100 μM) or mock-treated (VC) thereafter. Intracellular virus-specific mRNA and vRNA were quantified at 3 or 6 h p.i. (c) Inhibitory effect of ANA-0 to viral polymerase activity was tested by a mini-replicon assay. 293 T cells were transfected with plasmids encoding PB1, PB2, PA, NP genes, a firefly luciferase reporter-gene plasmid and an eGFP plasmid for transfection efficiency normalization. Indicated concentrations of ANA-0 were added at 5 h post-transfection. Luminescence and fluorescence were determined at 24 h post-transfection, respectively. The experiments were carried out in triplicate and repeated twice. The results are presented as mean values ± SD. Differences between groups were compared and analyzed using a one-way ANOVA. **indicates P < 0.01 as compared with the mock-treated control.